Table 1.
Optimization of reaction conditions for asymmetric Büchner reaction of N-propargyl ynamide 1a
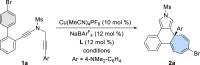 | ||||
|---|---|---|---|---|
| Entry | L | Reaction conditions | Yield (%)a | Ee (%)b |
| 1 | L1 | DCM, 35 °C, 0.5 h | 86 | 49 |
| 2 | L2 | DCM, 35 °C, 0.5 h | 83 | 55 |
| 3 | L3 | DCM, 35 °C, 0.5 h | 71 | <5 |
| 4 | L4 | DCM, 35 °C, 0.5 h | 79 | 11 |
| 5 | L5 | DCM, 35 °C, 0.5 h | 90 | 42 |
| 6 | L6 | DCM, 35 °C, 0.5 h | 94 | 79 |
| 7 | L7 | DCM, 35 °C, 0.5 h | 93 | 41 |
| 8 | L8 | DCM, 35 °C, 0.5 h | 86 | 51 |
| 9 | L9 | DCM, 35 °C, 0.5 h | 95 | 81 |
| 10 | L9 | DCE, 35 °C, 0.5 h | 94 | 84 |
| 11 | L9 | toluene, 35 °C, 0.5 h | 94 | 85 |
| 12 | L9 | THF, 35 °C, 0.5 h | 95 | 70 |
| 13 | L9 | toluene, 0 °C, 8 h | 93 | 87 |
| 14 | L9 | toluene, −20 °C, 40 h | 95 | 96 |
Reaction conditions: 1a (0.05 mmol), Cu(MeCN)4PF6 (0.005 mmol), L (0.006 mmol), NaBArF4 (0.006 mmol), solvent (1 mL), in Schlenk tubes.
aMeasured by 1H NMR using diethyl phthalate as internal standard.
bDetermined by HPLC analysis. Ms = methanesulfonyl, Mes = mesityl, NaBArF4 = sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate, DCM = dichloromethane, DCE = 1,2-dichloroethane, THF = tetrahydrofuran.
