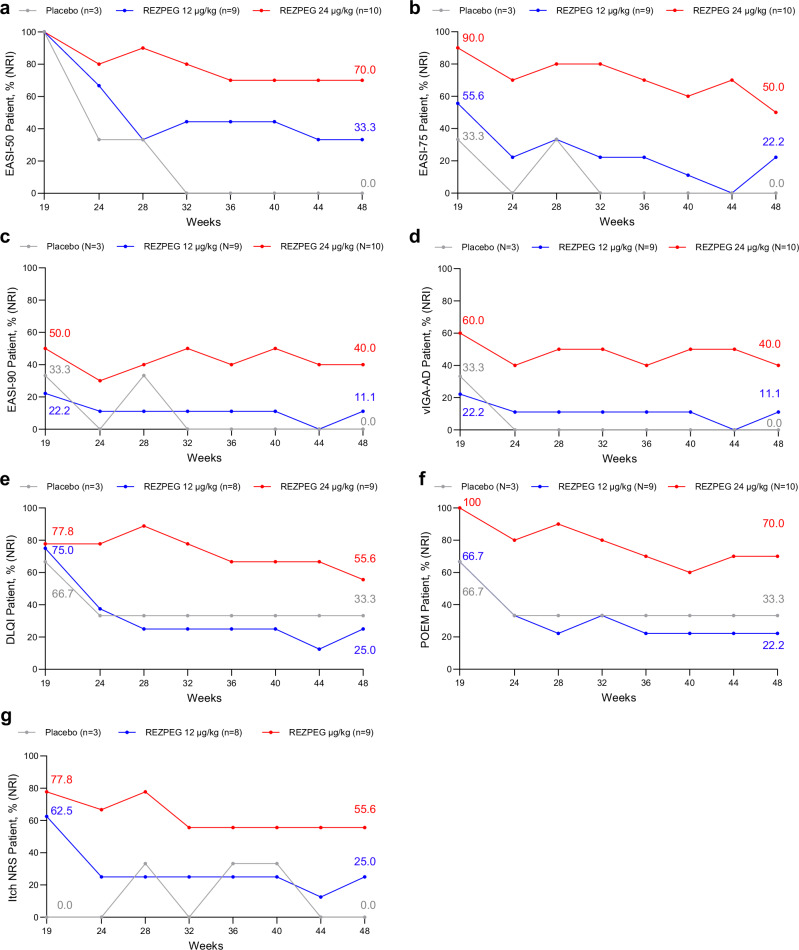
Fig. 5. AD off-treatment extended efficacy outcomes through week 48.
Proportion of week 19 EASI-50 responders maintaining a, EASI-50, decrease in EASI score by at least 50% relative to baseline; b, EASI-75, decrease in EASI score by at least 75% relative to baseline; c, EASI-90, decrease in EASI score by at least 90% relative to baseline; d, vIGA-AD score of 0 or 1 and a ≥ 2-point improvement from baseline; e DLQI score reduction by ≥ 4 points among patients with baseline score ≥ 4. f POEM score reduction by ≥ 4 points among patients with baseline score ≥ 4. g itch NRS score reduction by ≥ 4 points among patients with baseline score ≥ 4. Red, REZPEG 24 µg/kg; blue, REZPEG 12 µg/kg; grey, placebo. DLQI, Dermatology Life Quality Index; NRI, non-responder imputation; NRS, numeric rating scale; POEM, Patient-Oriented Eczema Measure; vIGA-AD, Validated Investigator Global Assessment for Atopic Dermatitis. Source data are provided as a Source Data file.