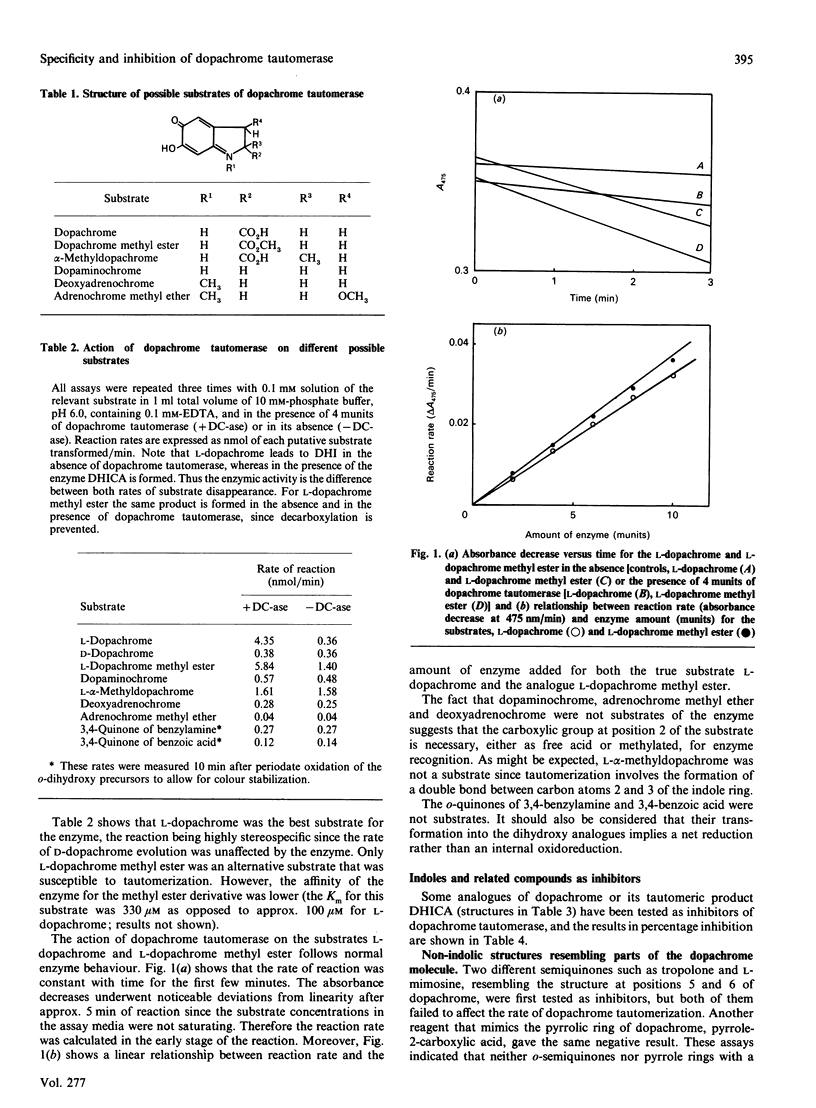
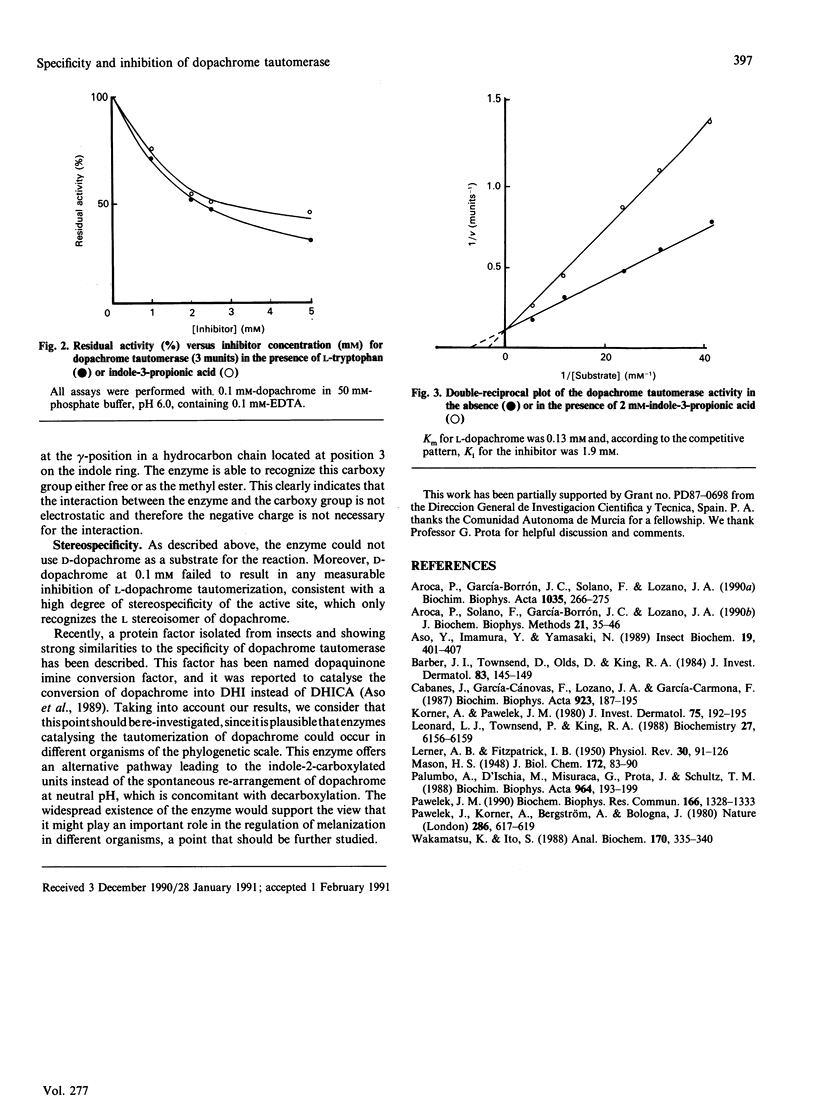
Abstract
Dopachrome tautomerase (EC 5.3.2.3) catalyses the tautomerization of dopachrome to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) within the melanin-formation pathway. We have analysed a series of substrate analogues and related compounds as possible substrates and inhibitors of tautomerization. The enzyme appears to be highly specific since D-dopachrome, alpha-methyldopachrome, dopaminochrome, adrenochrome methyl ether and deoxyadrenochrome are not substrates. Conversely, dopachrome tautomerase catalyses the tautomerization of dopachrome methyl ester, suggesting that a carboxy group, either free or as a methyl ester, is essential for enzyme recognition. No inhibition of dopachrome tautomerization was observed in the presence of either semiquinonic compounds, such as tropolone and L-mimosine, or pyrrole-2-carboxylic acid and unsubstituted indole. However, a number of indole derivatives, including DHICA, the product of dopachrome tautomerization, and the analogues 5-hydroxyindole-2-carboxylic and indole-2-carboxylic acid were able to inhibit the enzyme. Furthermore, indoles with a side chain at position 3 of the ring and containing a carboxylic group at the gamma-position of this chain, such as L-tryptophan or indole-3-propionic acid, are stronger inhibitors of the enzyme. Indole-3-carboxylic acid, indole-3-acetic acid and indole-3-butyric acid are very weak inhibitors, showing that the carboxylic group needs to be located at an optimal distance from the indole ring to mimic the carboxylic group at position 2 on the authentic substrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aroca P., Garcia-Borron J. C., Solano F., Lozano J. A. Regulation of mammalian melanogenesis. I: Partial purification and characterization of a dopachrome converting factor: dopachrome tautomerase. Biochim Biophys Acta. 1990 Sep 14;1035(3):266–275. doi: 10.1016/0304-4165(90)90088-e. [DOI] [PubMed] [Google Scholar]
- Aroca P., Solano F., García-Borrón J. C., Lozano J. A. A new spectrophotometric assay for dopachrome tautomerase. J Biochem Biophys Methods. 1990 Jun;21(1):35–46. doi: 10.1016/0165-022x(90)90043-c. [DOI] [PubMed] [Google Scholar]
- Barber J. I., Townsend D., Olds D. P., King R. A. Dopachrome oxidoreductase: a new enzyme in the pigment pathway. J Invest Dermatol. 1984 Aug;83(2):145–149. doi: 10.1111/1523-1747.ep12263381. [DOI] [PubMed] [Google Scholar]
- Cabanes J., García-Cánovas F., Lozano J. A., García-Carmona F. A kinetic study of the melanization pathway between L-tyrosine and dopachrome. Biochim Biophys Acta. 1987 Feb 20;923(2):187–195. doi: 10.1016/0304-4165(87)90003-1. [DOI] [PubMed] [Google Scholar]
- Körner A. M., Pawelek J. Dopachrome conversion: a possible control point in melanin biosynthesis. J Invest Dermatol. 1980 Aug;75(2):192–195. doi: 10.1111/1523-1747.ep12522650. [DOI] [PubMed] [Google Scholar]
- LERNER A. B., FITZPATRICK T. B. Biochemistry of melanin formation. Physiol Rev. 1950 Jan;30(1):91–126. doi: 10.1152/physrev.1950.30.1.91. [DOI] [PubMed] [Google Scholar]
- Leonard L. J., Townsend D., King R. A. Function of dopachrome oxidoreductase and metal ions in dopachrome conversion in the eumelanin pathway. Biochemistry. 1988 Aug 9;27(16):6156–6159. doi: 10.1021/bi00416a049. [DOI] [PubMed] [Google Scholar]
- Palumbo A., d'Ischia M., Misuraca G., Prota G., Schultz T. M. Structural modifications in biosynthetic melanins induced by metal ions. Biochim Biophys Acta. 1988 Feb 17;964(2):193–199. doi: 10.1016/0304-4165(88)90166-3. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M. Dopachrome conversion factor functions as an isomerase. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1328–1333. doi: 10.1016/0006-291x(90)91011-g. [DOI] [PubMed] [Google Scholar]
- Pawelek J., Körner A., Bergstrom A., Bologna J. New regulators of melanin biosynthesis and the autodestruction of melanoma cells. Nature. 1980 Aug 7;286(5773):617–619. doi: 10.1038/286617a0. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K., Ito S. Preparation of eumelanin-related metabolites 5,6-dihydroxyindole, 5,6-dihydroxyindole-2-carboxylic acid, and their O-methyl derivatives. Anal Biochem. 1988 May 1;170(2):335–340. doi: 10.1016/0003-2697(88)90639-2. [DOI] [PubMed] [Google Scholar]


