Abstract
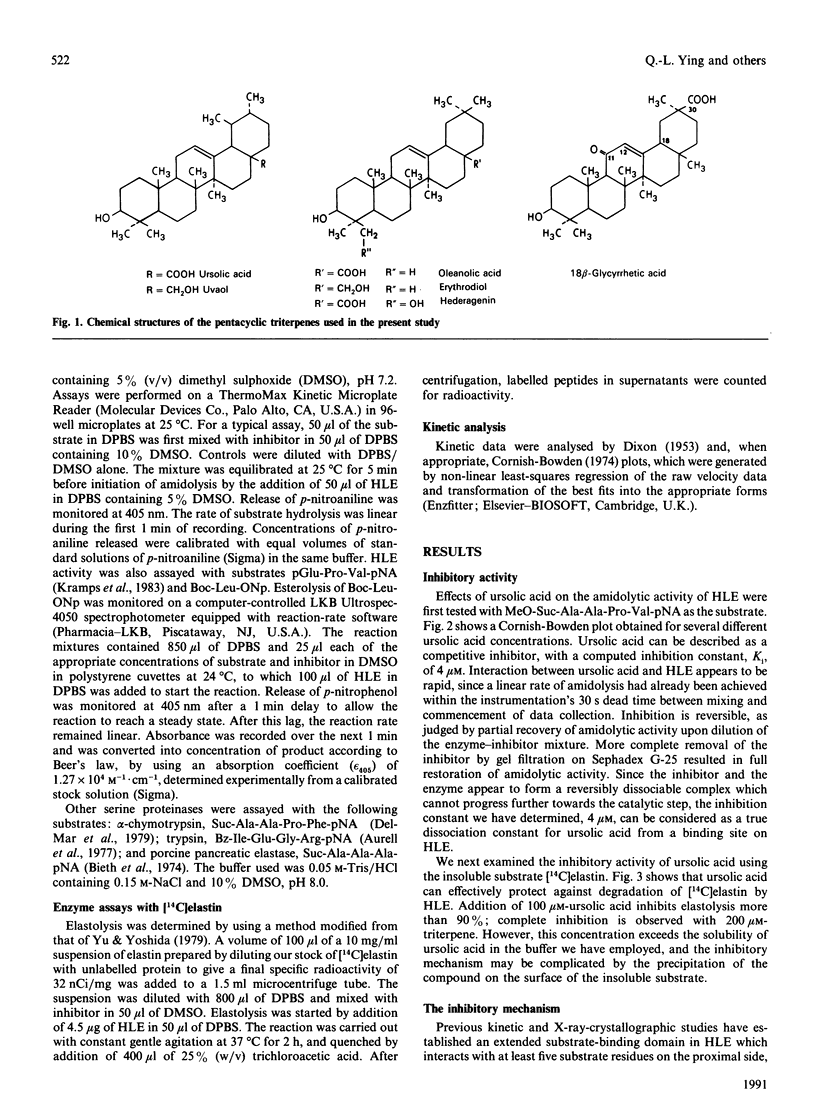
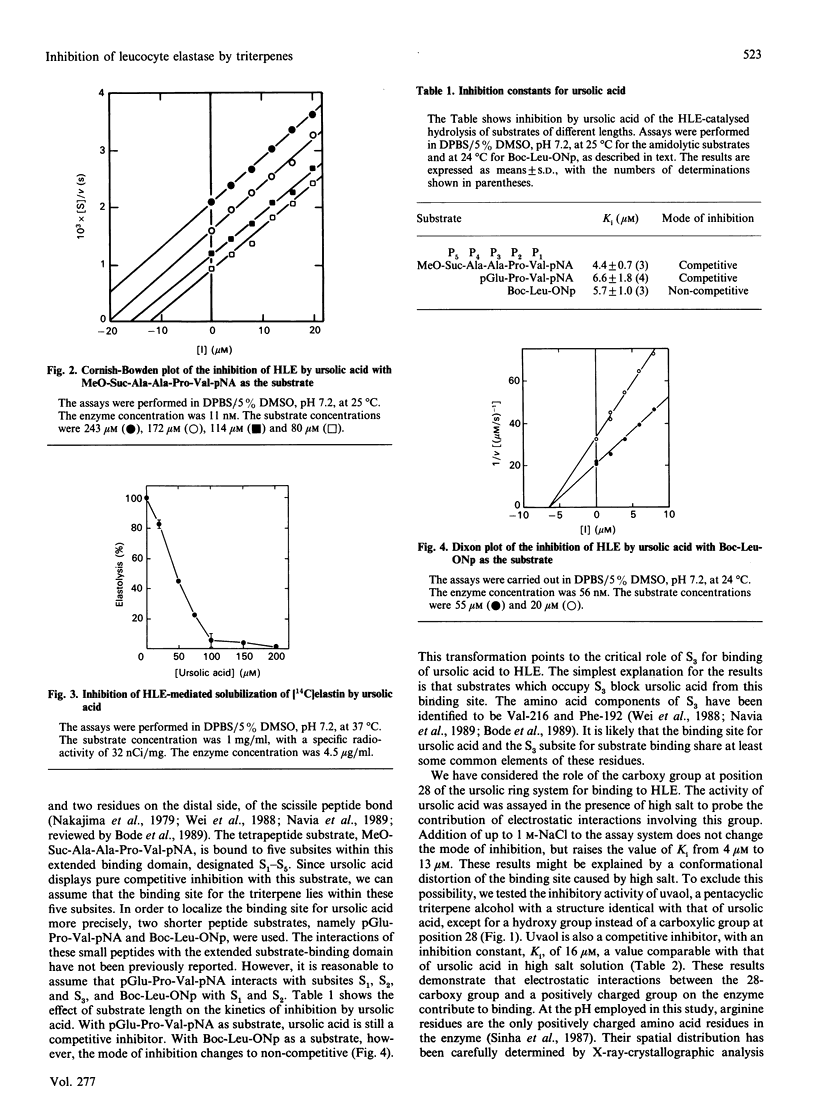
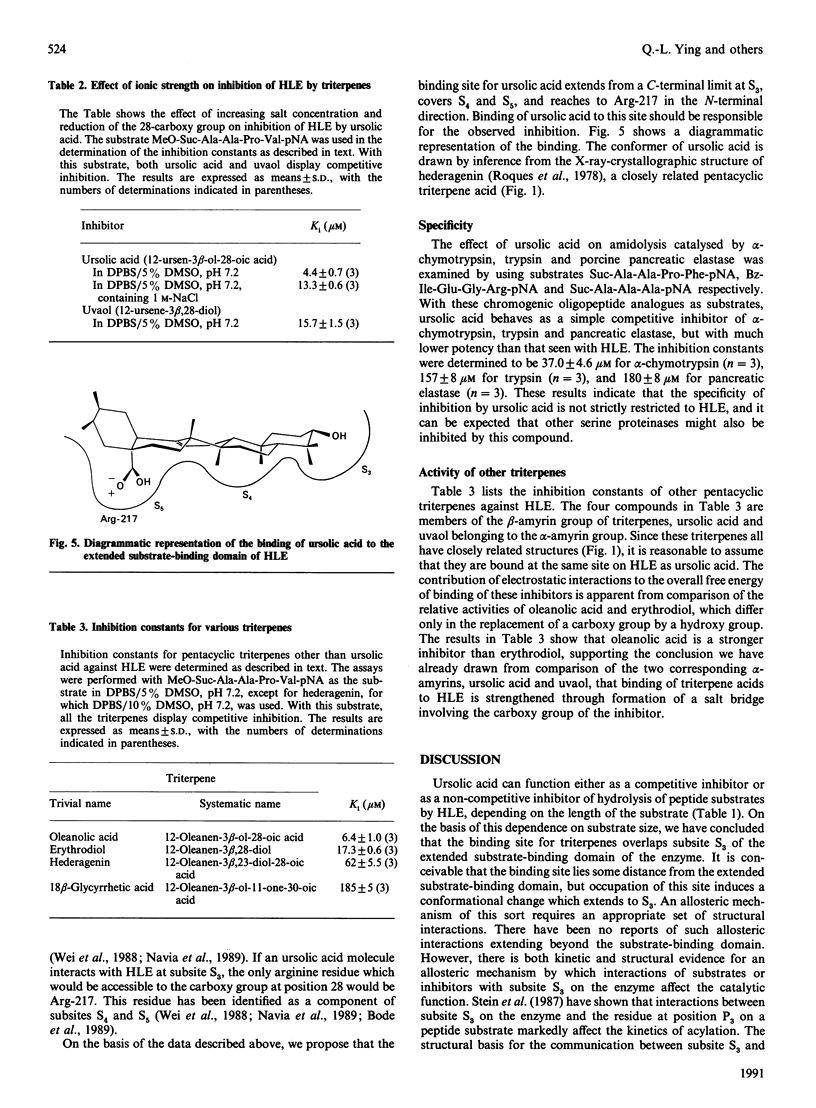
Several pentacyclic triterpenoid metabolites of plant origin are inhibitors of hydrolysis of both synthetic peptide substrates and elastin by human leucocyte elastase (HLE). Ursolic acid, the most potent of these compounds, has an inhibition constant of 4-6 microM for hydrolysis of peptide substrates in phosphate-buffered saline. With tripeptide and tetrapeptide substrates, the inhibition is purely competitive, whereas with a shorter dipeptide substrate the inhibition is non-competitive, suggesting that ursolic acid interacts with subsite S3 of the extended substrate-binding domain in HLE, but not with subsites S1 and S2. The carboxy group at position 28 in the pentacyclic-ring system of the triterpenes contributes to binding to HLE, since replacement of this group with a hydroxy group, as in uvaol, the alcohol analogue of ursolic acid, reduces the potency of inhibition. The inhibitory potency of ursolic acid is also reduced by addition of 1 M-NaCl, further supporting a postulated electrostatic interaction between the negative charge on the triterpene and a positively charged residue on the enzyme, which we assign to the side chain of Arg-217, located in the vicinity of subsites S4 and S5 in HLE. These observations are consistent with a binding site for ursolic acid which extends from S3 towards S4 and S5 on the enzyme. Other triterpenes, including oleanolic acid, erythrodiol, hederagenin and 18 beta-glycyrrhetic acid, can also interact with this binding site. On the basis of these results we conclude that the extended substrate-binding domain of HLE can accommodate a variety of hydrophobic ligands, including not only such molecules as fatty acids [Ashe & Zimmerman (1977) Biochem. Biophys. Res. Commun. 75, 194-199; Cook & Ternai (1988) Biol. Chem. Hoppe-Seyler 369, 629-637], but also polycyclic molecules such as the pentacyclic triterpenoids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurell L., Friberger P., Karlsson G., Claeson G. A new sensitive and highly specific chromogenic peptide substrate for factor Xa. Thromb Res. 1977 Nov;11(5):595–609. doi: 10.1016/0049-3848(77)90018-4. [DOI] [PubMed] [Google Scholar]
- Bhargava K. P., Gupta M. B., Gupta G. P., Mitra C. R. Anti-inflammatory activity of saponins and ot-her natural products. Indian J Med Res. 1970 Jun;58(6):724–730. [PubMed] [Google Scholar]
- Bieth J. G. In vivo significance of kinetic constants of protein proteinase inhibitors. Biochem Med. 1984 Dec;32(3):387–397. doi: 10.1016/0006-2944(84)90046-2. [DOI] [PubMed] [Google Scholar]
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Bode W., Meyer E., Jr, Powers J. C. Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry. 1989 Mar 7;28(5):1951–1963. doi: 10.1021/bi00431a001. [DOI] [PubMed] [Google Scholar]
- Chaturvedi A. K., Parmar S. S., Nigam S. K., Bhatnagar S. C., Misra G., Sastry B. V. Anti-inflammatory and anticonvulsant properties of some natural plant triterpenoids. Pharmacol Res Commun. 1976 Apr;8(2):199–210. doi: 10.1016/0031-6989(76)90009-6. [DOI] [PubMed] [Google Scholar]
- Cook L., Ternai B. Similar binding sites for unsaturated fatty acids and alkyl 2-pyrone inhibitors of human sputum elastase. Biol Chem Hoppe Seyler. 1988 Jul;369(7):627–631. doi: 10.1515/bchm3.1988.369.2.627. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem J. 1974 Jan;137(1):143–144. doi: 10.1042/bj1370143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelMar E. G., Largman C., Brodrick J. W., Geokas M. C. A sensitive new substrate for chymotrypsin. Anal Biochem. 1979 Nov 1;99(2):316–320. doi: 10.1016/s0003-2697(79)80013-5. [DOI] [PubMed] [Google Scholar]
- Dewald B., Rindler-Ludwig R., Bretz U., Baggiolini M. Subcellular localization and heterogeneity of neutral proteases in neutrophilic polymorphonuclear leukocytes. J Exp Med. 1975 Apr 1;141(4):709–723. doi: 10.1084/jem.141.4.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINNEY R. S., SOMERS G. F. The antiinflammatory activity of glycyrrhetinic acid and derivatives. J Pharm Pharmacol. 1958 Oct;10(10):613–620. doi: 10.1111/j.2042-7158.1958.tb10349.x. [DOI] [PubMed] [Google Scholar]
- Groutas W. C. Inhibitors of leukocyte elastase and leukocyte cathepsin G. Agents for the treatment of emphysema and related ailments. Med Res Rev. 1987 Apr-Jun;7(2):227–241. doi: 10.1002/med.2610070205. [DOI] [PubMed] [Google Scholar]
- Gupta M. B., Bhalla T. N., Gupta G. P., Mitra C. R., Bhargava K. P. Anti-inflammatory activity of natural products. I. Triterpenoids. Eur J Pharmacol. 1969 Apr;6(1):67–70. doi: 10.1016/0014-2999(69)90067-3. [DOI] [PubMed] [Google Scholar]
- Gupta M. B., Nath R., Gupta G. P., Bhargava K. P. Antiulcer activity of some plant triterpenoids. Indian J Med Res. 1981 Apr;73:649–652. [PubMed] [Google Scholar]
- Janoff A. Elastase in tissue injury. Annu Rev Med. 1985;36:207–216. doi: 10.1146/annurev.me.36.020185.001231. [DOI] [PubMed] [Google Scholar]
- Kosuge T., Yokota M., Sugiyama K., Mure T., Yamazawa H., Yamamoto T. Studies on bioactive substances in crude drugs used for arthritic diseases in traditional Chinese medicine. III. Isolation and identification of anti-inflammatory and analgesic principles from the whole herb of Pyrola rotundifolia L. Chem Pharm Bull (Tokyo) 1985 Dec;33(12):5355–5357. doi: 10.1248/cpb.33.5355. [DOI] [PubMed] [Google Scholar]
- Kramps J. A., van Twisk C., van der Linden A. C. L-Pyroglutamyl-L-prolyl-L-valine-p-nitroanilide, a highly specific substrate for granulocyte elastase. Scand J Clin Lab Invest. 1983 Sep;43(5):427–432. [PubMed] [Google Scholar]
- Nakajima K., Powers J. C., Ashe B. M., Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J Biol Chem. 1979 May 25;254(10):4027–4032. [PubMed] [Google Scholar]
- Navia M. A., McKeever B. M., Springer J. P., Lin T. Y., Williams H. R., Fluder E. M., Dorn C. P., Hoogsteen K. Structure of human neutrophil elastase in complex with a peptide chloromethyl ketone inhibitor at 1.84-A resolution. Proc Natl Acad Sci U S A. 1989 Jan;86(1):7–11. doi: 10.1073/pnas.86.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J. C., Boone R., Carroll D. L., Gupton B. F., Kam C. M., Nishino N., Sakamoto M., Tuhy P. M. Reaction of azapeptides with human leukocyte elastase and porcine pancreatic elastase. New inhibitors and active site titrants. J Biol Chem. 1984 Apr 10;259(7):4288–4294. [PubMed] [Google Scholar]
- Powers J. C. Synthetic elastase inhibitors: prospects for use in the treatment of emphysema. Am Rev Respir Dis. 1983 Feb;127(2):S54–S58. doi: 10.1164/arrd.1983.127.2P2.S54. [DOI] [PubMed] [Google Scholar]
- Sinha S., Watorek W., Karr S., Giles J., Bode W., Travis J. Primary structure of human neutrophil elastase. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2228–2232. doi: 10.1073/pnas.84.8.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soskel N. T., Watanabe S., Hardie R., Shenvi A. B., Punt J. A., Kettner C. Effects of dosage and timing of administration of a peptide boronic acid inhibitor on lung mechanics and morphometrics in elastase-induced emphysema in hamsters. Am Rev Respir Dis. 1986 Apr;133(4):635–638. doi: 10.1164/arrd.1986.133.4.635. [DOI] [PubMed] [Google Scholar]
- Stein R. L., Strimpler A. M., Hori H., Powers J. C. Catalysis by human leukocyte elastase: mechanistic insights into specificity requirements. Biochemistry. 1987 Mar 10;26(5):1301–1305. doi: 10.1021/bi00379a015. [DOI] [PubMed] [Google Scholar]
- Takagi K., Park E. H., Kato H. Anti-inflammatory activities of hederagenin and crude saponin isolated from Sapindus mukorossi Gaertn. Chem Pharm Bull (Tokyo) 1980;28(4):1183–1188. doi: 10.1248/cpb.28.1183. [DOI] [PubMed] [Google Scholar]
- Wei A. Z., Mayr I., Bode W. The refined 2.3 A crystal structure of human leukocyte elastase in a complex with a valine chloromethyl ketone inhibitor. FEBS Lett. 1988 Jul 18;234(2):367–373. doi: 10.1016/0014-5793(88)80118-2. [DOI] [PubMed] [Google Scholar]
- Yu S. Y., Yoshida A. Amorphous [14C]elastin as a substrate for assaying elastolytic enzyme in cellular and tissue extracts. Biochem Med. 1979 Feb;21(1):108–120. doi: 10.1016/0006-2944(79)90062-0. [DOI] [PubMed] [Google Scholar]


