Abstract
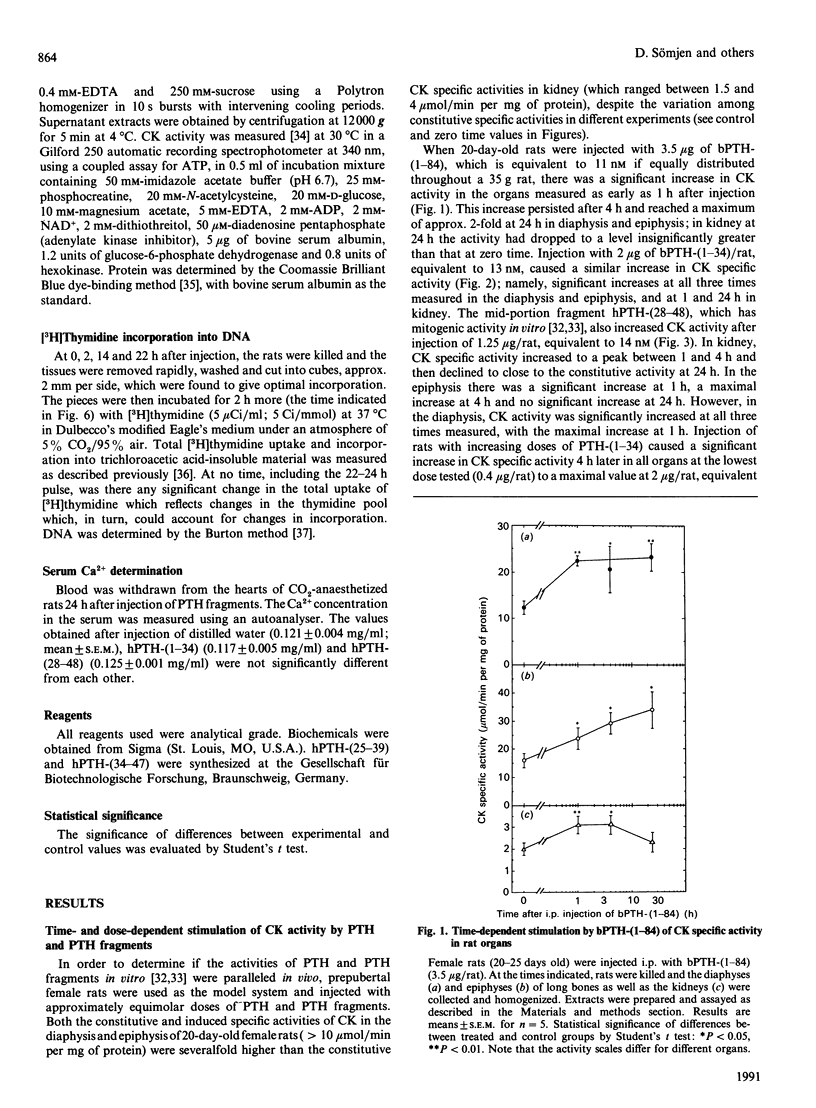
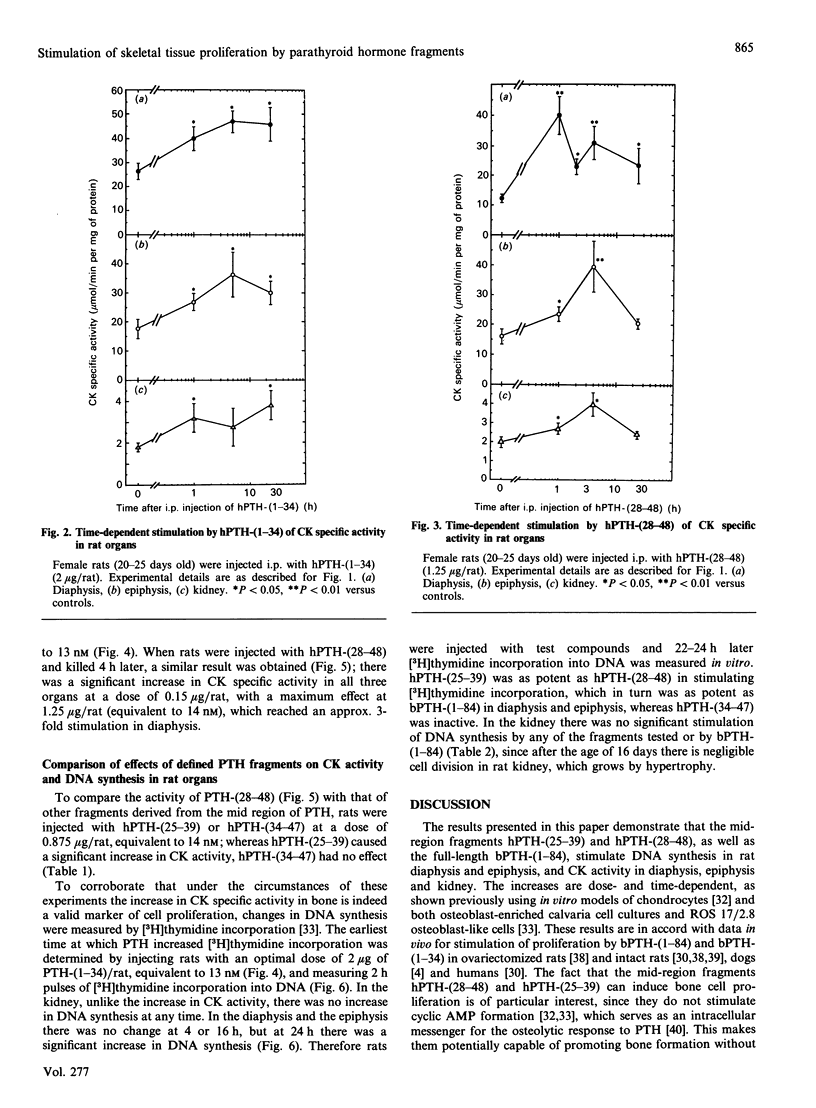
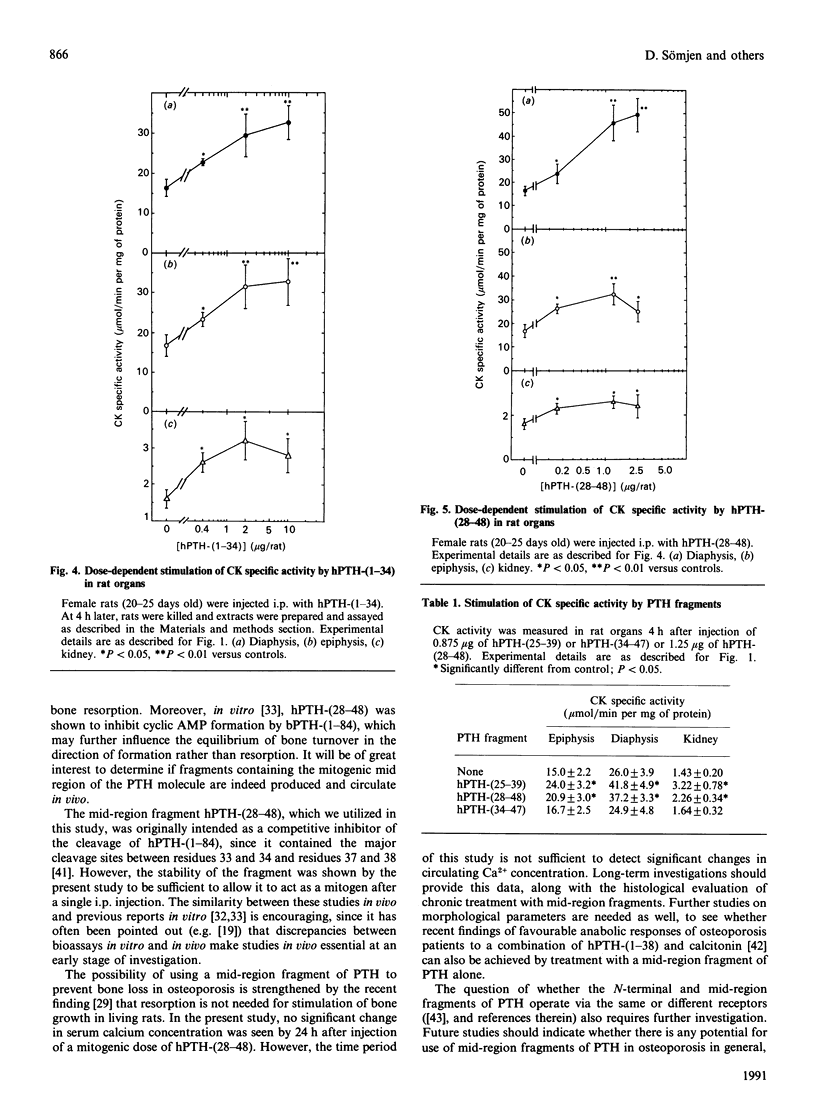
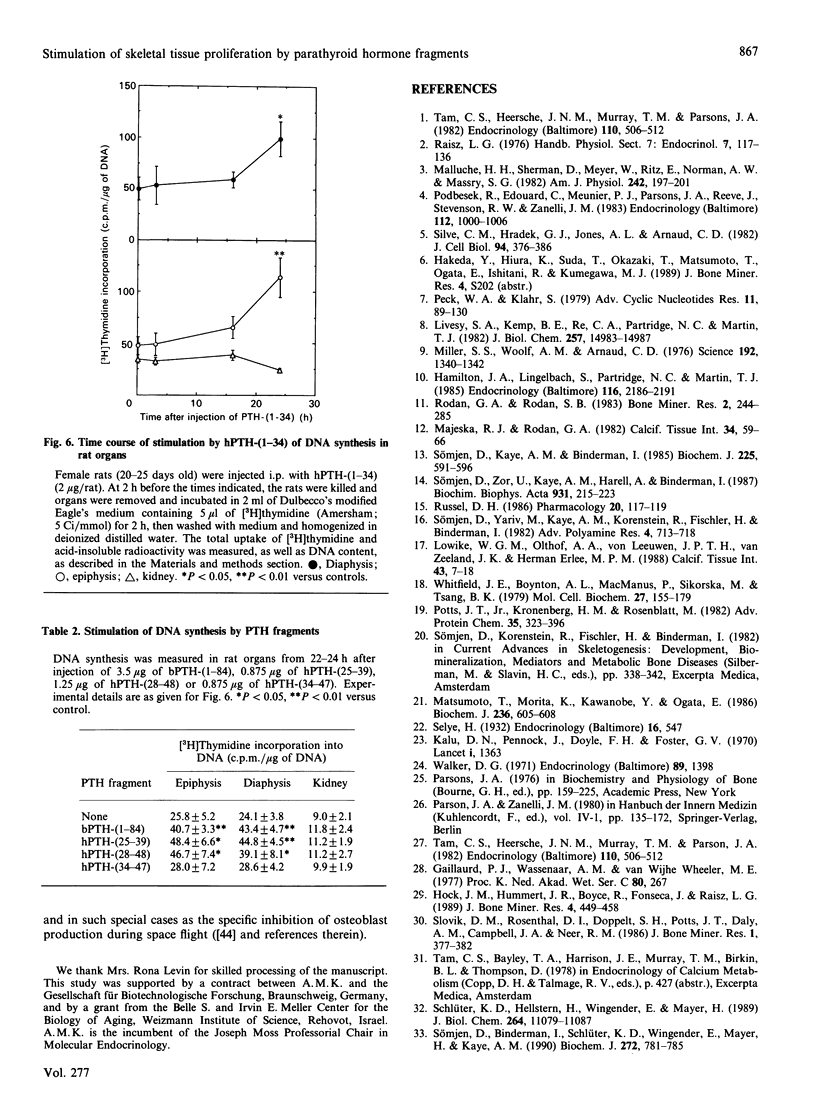
We have found, in previous studies in vitro using skeletal derived cell cultures, that mid-region fragments of human parathyroid hormone (hPTH) stimulate [3H]thymidine incorporation into DNA and increase the specific activity of the brain-type isoenzyme of creatine kinase (CK). These changes occurred without an increase in cyclic AMP formation which is linked to bone resorption. In this study, we found that the mid-region fragment hPTH-(28-48) stimulated CK activity in diaphysis, epiphysis and kidney in a time- and dose-dependent manner, parallel to the effects of the whole molecule bovine (b)PTH-(1-84) and the fully active fragment hPTH-(1-34). The increase caused by hPTH-(28-48) at a dose of 1.25 micrograms/rat was not less than the 2-fold increase caused by a roughly equimolar concentration bPTH-(1-84). A significant increase was reached at 1 h after intraperitoneal injection in all cases. All three sequences of PTH caused an increase in [3H]thymidine incorporation into DNA in diaphysis and epiphysis, but not in kidney, 24 h after injection. A fragment further towards the C-terminal, hPTH-(34-47), was inactive compared with an equimolar concentration of the fragment hPTH-(25-39), which stimulated both CK activity and DNA synthesis. These results in vivo are in line with previous findings in vitro; they provide further support for the suggestion that mid-region fragments of the PTH molecule could be used to induce bone formation without incurring the deleterious effect of bone resorption.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Garetto L. P., Gonsalves M. R., Morey E. R., Durnova G., Roberts W. E. Preosteoblast production 55 hours after a 12.5-day spaceflight on Cosmos 1887. FASEB J. 1990 Jan;4(1):24–28. doi: 10.1096/fasebj.4.1.2295374. [DOI] [PubMed] [Google Scholar]
- Gunness-Hey M., Hock J. M. Increased trabecular bone mass in rats treated with human synthetic parathyroid hormone. Metab Bone Dis Relat Res. 1984;5(4):177–181. doi: 10.1016/0221-8747(84)90026-2. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Lingelbach S., Partridge N. C., Martin T. J. Regulation of plasminogen activator production by bone-resorbing hormones in normal and malignant osteoblasts. Endocrinology. 1985 Jun;116(6):2186–2191. doi: 10.1210/endo-116-6-2186. [DOI] [PubMed] [Google Scholar]
- Hesch R. D., Busch U., Prokop M., Delling G., Rittinghaus E. F. Increase of vertebral density by combination therapy with pulsatile 1-38hPTH and sequential addition of calcitonin nasal spray in osteoporotic patients. Calcif Tissue Int. 1989 Mar;44(3):176–180. doi: 10.1007/BF02556561. [DOI] [PubMed] [Google Scholar]
- Hock J. M., Hummert J. R., Boyce R., Fonseca J., Raisz L. G. Resorption is not essential for the stimulation of bone growth by hPTH-(1-34) in rats in vivo. J Bone Miner Res. 1989 Jun;4(3):449–458. doi: 10.1002/jbmr.5650040321. [DOI] [PubMed] [Google Scholar]
- Hori M., Uzawa T., Morita K., Noda T., Takahashi H., Inoue J. Effect of human parathyroid hormone (PTH(1-34)) on experimental osteopenia of rats induced by ovariectomy. Bone Miner. 1988 Jan;3(3):193–199. [PubMed] [Google Scholar]
- Kalu D. N., Doyle F. H., Pennock J., Foster G. V. Parathyroid hormone and experimental osteosclerosis. Lancet. 1970 Jun 27;1(7661):1363–1366. doi: 10.1016/s0140-6736(70)91271-7. [DOI] [PubMed] [Google Scholar]
- Klein R. F., Nissenson R. A., Strewler G. J. Forskolin mimics the effects of calcitonin but not parathyroid hormone on bone resorption in vitro. Bone Miner. 1988 Jul;4(3):247–256. [PubMed] [Google Scholar]
- Livesey S. A., Kemp B. E., Re C. A., Partridge N. C., Martin T. J. Selective hormonal activation of cyclic AMP-dependent protein kinase isoenzymes in normal and malignant osteoblasts. J Biol Chem. 1982 Dec 25;257(24):14983–14987. [PubMed] [Google Scholar]
- Löwik C. W., Olthof A. A., van Leeuwen J. P., van Zeeland J. K., Herrmann-Erlee M. P. Induction of ornithine decarboxylase activity in isolated chicken osteoblasts by parathyroid hormone: the role of cAMP and calcium. Calcif Tissue Int. 1988 Jul;43(1):7–18. doi: 10.1007/BF02555162. [DOI] [PubMed] [Google Scholar]
- Majeska R. J., Rodan G. A. Alkaline phosphatase inhibition by parathyroid hormone and isoproterenol in a clonal rat osteosarcoma cell line. Possible mediation by cyclic AMP. Calcif Tissue Int. 1982 Jan;34(1):59–66. doi: 10.1007/BF02411210. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Morita K., Kawanobe Y., Ogata E. Effect of parathyroid hormone on phospholipid metabolism in osteoblast-like rat osteogenic sarcoma cells. Biochem J. 1986 Jun 1;236(2):605–608. doi: 10.1042/bj2360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. S., Wolf A. M., Arnaud C. D. Bone cells in culture: morphologic transformation by hormones. Science. 1976 Jun 25;192(4246):1340–1343. doi: 10.1126/science.1273593. [DOI] [PubMed] [Google Scholar]
- Peck W. A., Klahr S. Cyclic nucleotides in bone and mineral metabolism. Adv Cyclic Nucleotide Res. 1979;11:89–130. [PubMed] [Google Scholar]
- Podbesek R., Edouard C., Meunier P. J., Parsons J. A., Reeve J., Stevenson R. W., Zanelli J. M. Effects of two treatment regimes with synthetic human parathyroid hormone fragment on bone formation and the tissue balance of trabecular bone in greyhounds. Endocrinology. 1983 Mar;112(3):1000–1006. doi: 10.1210/endo-112-3-1000. [DOI] [PubMed] [Google Scholar]
- Potts J. T., Jr, Kronenberg H. M., Rosenblatt M. Parathyroid hormone: chemistry, biosynthesis, and mode of action. Adv Protein Chem. 1982;35:323–396. doi: 10.1016/s0065-3233(08)60471-4. [DOI] [PubMed] [Google Scholar]
- Reiss N. A., Kaye A. M. Identification of the major component of the estrogen-induced protein of rat uterus as the BB isozyme of creatine kinase. J Biol Chem. 1981 Jun 10;256(11):5741–5749. [PubMed] [Google Scholar]
- Rosenblatt M., Segre G. V., Potts J. T., Jr Synthesis of a fragment of parathyroid hormone, bPTH-(28-48): an inhibitor of hormone cleavage in vivo. Biochemistry. 1977 Jun 28;16(13):2811–2816. doi: 10.1021/bi00632a001. [DOI] [PubMed] [Google Scholar]
- Seitz P. K., Nickols G. A., Nickols M. A., McPherson M. B., Cooper C. W. Radioiodinated rat parathyroid hormone-(1-34) binds to its receptor on rat osteosarcoma cells in a manner consistent with two classes of binding sites. J Bone Miner Res. 1990 Apr;5(4):353–359. doi: 10.1002/jbmr.5650050408. [DOI] [PubMed] [Google Scholar]
- Silve C. M., Hradek G. T., Jones A. L., Arnaud C. D. Parathyroid hormone receptor in intact embryonic chicken bone: characterization and cellular localization. J Cell Biol. 1982 Aug;94(2):379–386. doi: 10.1083/jcb.94.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovik D. M., Rosenthal D. I., Doppelt S. H., Potts J. T., Jr, Daly M. A., Campbell J. A., Neer R. M. Restoration of spinal bone in osteoporotic men by treatment with human parathyroid hormone (1-34) and 1,25-dihydroxyvitamin D. J Bone Miner Res. 1986 Aug;1(4):377–381. doi: 10.1002/jbmr.5650010411. [DOI] [PubMed] [Google Scholar]
- Sömjen D., Binderman I., Schlüter K. D., Wingender E., Mayer H., Kaye A. M. Stimulation by defined parathyroid hormone fragments of cell proliferation in skeletal-derived cell cultures. Biochem J. 1990 Dec 15;272(3):781–785. doi: 10.1042/bj2720781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sömjen D., Binderman I., Weisman Y. The effects of 24R,25-dihydroxycholecalciferol and of 1 alpha,25-dihydroxycholecalciferol on ornithine decarboxylase activity and on DNA synthesis in the epiphysis and diaphysis of rat bone and in the duodenum. Biochem J. 1983 Aug 15;214(2):293–298. doi: 10.1042/bj2140293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sömjen D., Kaye A. M., Binderman I. Stimulation of creatine kinase BB activity by parathyroid hormone and by prostaglandin E2 in cultured bone cells. Biochem J. 1985 Feb 1;225(3):591–596. doi: 10.1042/bj2250591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sömjen D., Zor U., Kaye A. M., Harell A., Binderman I. Parathyroid hormone induction of creatine kinase activity and DNA synthesis is mimicked by phospholipase C, diacylglycerol and phorbol ester. Biochim Biophys Acta. 1987 Nov 12;931(2):215–223. doi: 10.1016/0167-4889(87)90209-6. [DOI] [PubMed] [Google Scholar]
- Tam C. S., Heersche J. N., Murray T. M., Parsons J. A. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982 Feb;110(2):506–512. doi: 10.1210/endo-110-2-506. [DOI] [PubMed] [Google Scholar]
- Tam C. S., Heersche J. N., Murray T. M., Parsons J. A. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982 Feb;110(2):506–512. doi: 10.1210/endo-110-2-506. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Boynton A. L., MacManus J. P., Sikorska M., Tsang B. K. The regulation of cell proliferation by calcium and cyclic AMP. Mol Cell Biochem. 1979 Nov 1;27(3):155–179. doi: 10.1007/BF00215364. [DOI] [PubMed] [Google Scholar]


