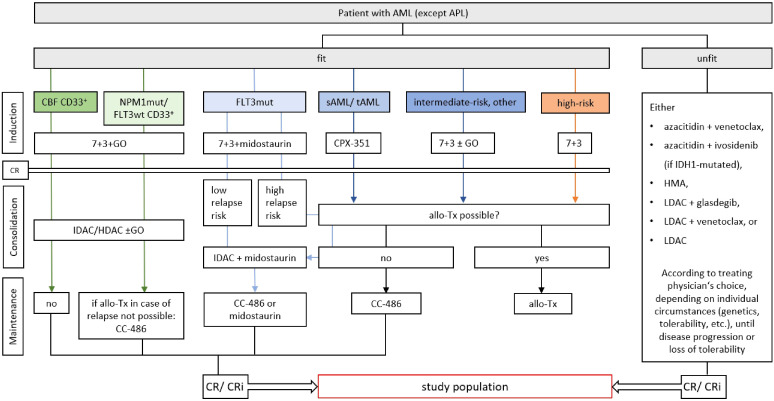
Figure 2.
Study population within the framework of current treatment guidelines of the medical societies in hematology and medical oncology of the German speaking countries (DGHO) (4). Study patients must have reached complete remission (CR) or CR with incomplete blood count recovery (CRi) with any type of first-line therapy. Allogeneic stem cell transplantation (allo-Tx) must not be a viable treatment option. AML, acute myeloid leukemia; APL, acute promyelocyte leukemia; 7 + 3, combinatorial treatment with cytarabine (7 days) and daunorubicin (3 days); CBF, core binding factor mutation; GO, gemtuzumab ozogamicin; mut, mutated; sAML, secondary AML; tAML, therapy-associated AML; LDAC, low-dose cytarabine; IDAC, intermediate-dose cytarabine; HDAC, high-dose cytarabine; CC-486, oral azacytidine; CPX-352, liposomal cytarabine and daunorubicin; HMA, hypomethylating agent.