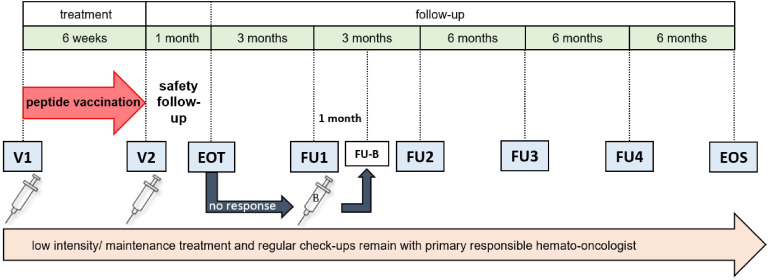
Figure 3.
Study schedule. AML patients receive two vaccine doses (V1 and V2), six weeks apart. After one month, an end-of-treatment (EOT) visit is scheduled, followed by two follow-up visits (FU1 and FU2), three months apart. Evaluation of immune response by IFN-γ ELISpot assay is done at every visit except screening. If immune response at EOT is insufficient, a booster vaccination (B) can be applied at FU1, which will then be followed by an additional follow-up visit (FU-B) after one month. After FU2, two more follow-up visits (FU3 and FU4) and an end of study (EOS) visit are performed, spaced six months apart. Throughout the study, any ongoing therapy and its monitoring are conducted by the primary responsible hematologist/oncologist. CR, complete remission; CRi, complete remission with incomplete blood count recovery; n, number.