Abstract
Introduction and importance
Renal transplant recipients have a higher risk for developing cancers compared to the general population due to high-dose immunosuppression. The risk of renal cell carcinoma (RCC) in native kidneys is 7-fold greater than the general population and development of RCC in an allograft kidney is extremely rare. We report the diagnosis and management of a large RCC in an allograft renal transplant and metastatic disease in a regional lymph node.
Case presentation
A 46 year old male patient with a history of simultaneous pancreas and kidney transplant presented with visible haematuria. His pancreas allograft continued to function well however following severe BK nephritis his renal transplant failed. A CT urogram demonstrated a 6 cm contrast enhancing mass in the failed renal transplant and an enlarged pelvic lymph node. He underwent a transplant nephrectomy with excision of the metastatic lymph node deposit.
Clinical discussion
We report the diagnosis and management of a large RCC in an allograft renal transplant and metastatic disease in a regional lymph node. There is currently no guidelines on the management of allograft RCC.
Conclusion
Our case report shows that surgical excision of a large RCC in an allograft renal transplant is possible.
Keywords: Kidney transplant, Pancreas transplant, Renal carcinoma, Case report
Highlights
-
•
Renal transplant recipients a higher risk for developing cancers
-
•
Developing an RCC in an allograft kidney is extremely rare.
-
•
We report a large RCC in an allograft transplant and metastatic disease in a regional lymph node.
-
•
A transplant nephrectomy with excision of the metastatic lymph node is described.
-
•
There are currently no guidelines on the management of allograft RCC.
1. Introduction
Renal transplant recipients have a higher risk for developing cancers compared to the general population due to high-dose immunosuppression. The risk of developing lymphoma is approximately 35 times greater than the general population [1]. In addition, renal transplant recipients <50 years, have a 200 fold increased risk for developing cutaneous cancer compared to an age-matched non-transplanted population 6 years post-renal transplant [2]. Overall, there is a 14 % risk for developing neoplasia within the first decade of renal transplantation and this risk increases to 40 % at 20 years post-transplant. Conversely, this compares to a 6 % cumulative incidence in an age-matched non-transplanted population [3]. Furthermore, transplant recipients have more aggressive cancer and poorer prognostic outcomes compared to the general population [4]. Finally, the risk of renal cell carcinoma (RCC) in native kidneys is 7-fold greater than the general population and [5] development of RCC in an allograft kidney is extremely rare. We report the diagnosis and management of a large RCC in an allograft renal transplant and metastatic disease in a regional lymph node.
2. Methods
Surgical Case Report (SCARE) guidelines were followed when writing this case report [6].
2.1. Case report
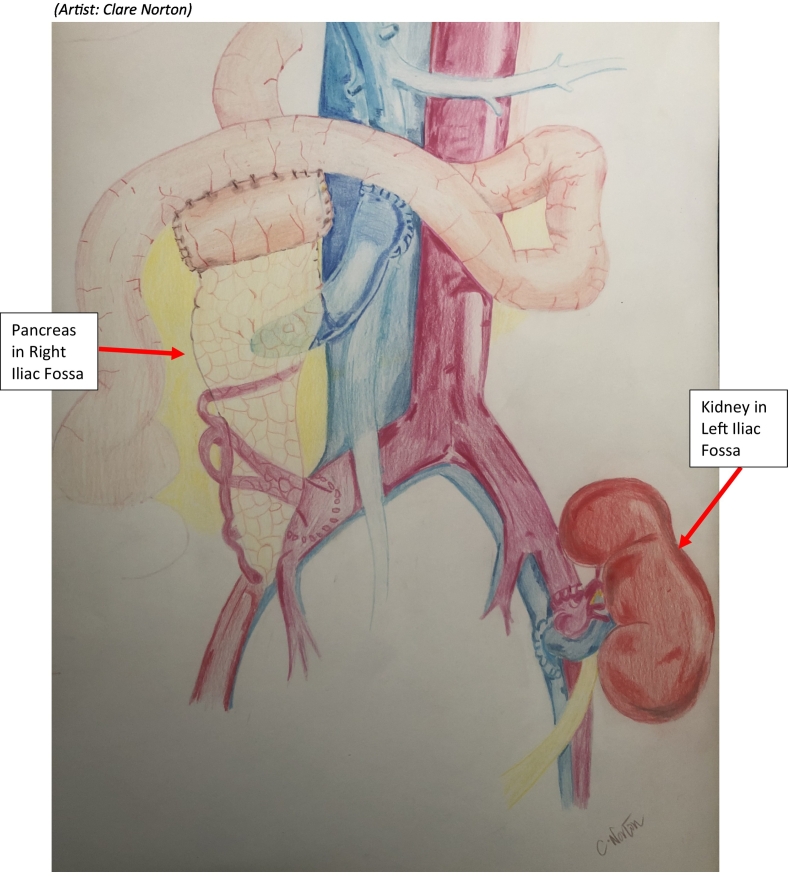
A 46 year old male patient presented with visible haematuria. He had no history of recent trauma, urinary tract infections (UTIs) and was a lifelong non-smoker. His relevant past surgical history was a simultaneous pancreas and kidney transplant in 2014 (see Drawing 1) due to insulin dependent diabetes mellitus (IDDM) for 25 years which resulted in diabetes mellitus (DM) induced nephropathy in 2013.
Drawing 1.
Simultaneous pancreas and renal transplantation, diagram of location of both organs.
(Artist: Clare Norton.)
Initially, there was excellent pancreas and renal allograft function. However, in 2015, he developed severe BK nephritis and was treated with a low dose antiviral, cidofovir 37.5 mg every two weeks and a reduced regime of immunosuppression of tacrolimus 2 mg twice daily (BD), azaithioprine 50 mg once daily (OD) and prednisolone 5 mg OD. He was previously on tacrolimus 8 mg BD, mycophenolate mofetil 500 mg BD and prednisolone 30 mg OD. Ultimately, persistent BK Virus resulted in failure of the renal transplant and he commenced haemodialysis in 2016. His pancreas transplant continued to have excellent endocrine and exocrine graft function.
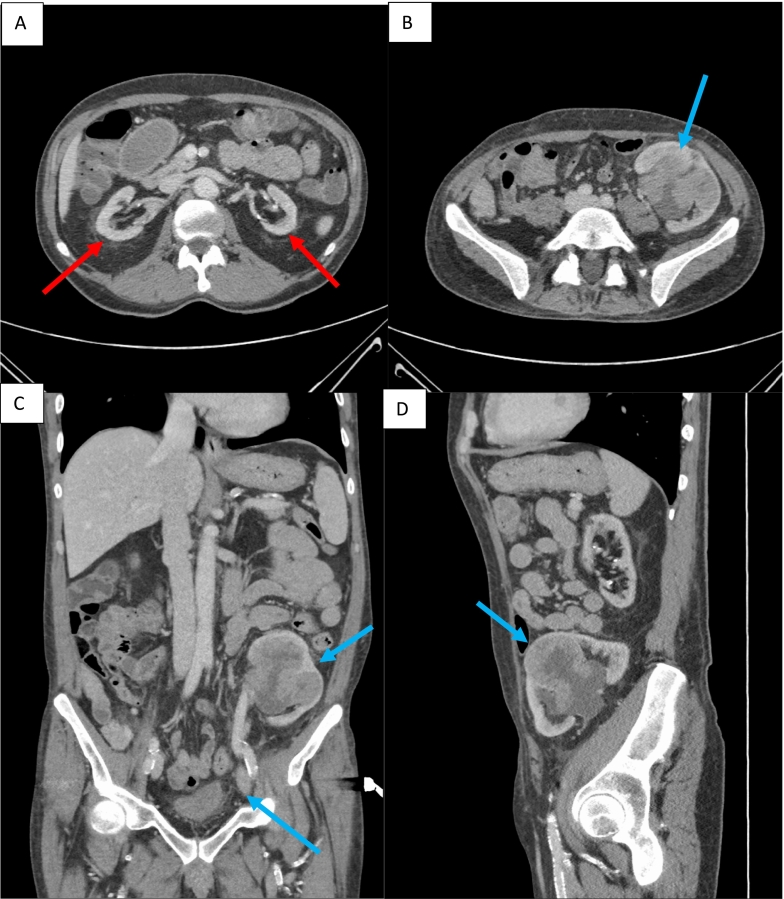
Following urgent review for visible haematuria, tenderness was noted on examination over his left iliac fossa, at the site of his previous renal transplant. He underwent a rigid cystoscopy and a Computed Tomography (CT) urogram. Rigid cystoscopy found no cause for bleeding however the CT urogram demonstrated a 6 cm contrast enhancing mass in the failed renal transplant and an enlarged external iliac lymph node (see Fig. 1A–D).
Fig. 1.
Images A–D (CT urogram): A) Axial slice showing native atrophic kidneys (red arrows) in arterial phase. B) Axial slice during arterial phase of CT showing 61 mm × 53 mm mass in the mid-anterior renal transplant with distended calyces and multifocal high density material within the collecting system (blue arrow), concerning for Transition Cell Carcinoma (TCC). C) Coronal slice of CT in arterial phase showing renal transplant lesion and lymph node (blue arrows). D) Sagittal slice of CT in arterial phase showing renal transplant lesion (blue arrow).
Following discussion at the multidisciplinary team meeting the differential for this transplant renal lesion was lymphoma, TCC or renal cell carcinoma and a renal biopsy was performed for diagnostic purposes. The patient underwent an open transplant nephrectomy. Care was taken to avoid injury to the functioning pancreas transplant.
Operative steps of a retroperitoneal extracapsular transplant nephrectomy: Drawing 1
-
1.
A Reisberg incision was made over the left iliac fossa at the location of the previously sited renal transplant.
-
2.
The renal tumour was identified in the midpole of the kidney extending into the renal hilum. The external iliac lymph node was also palpable.
-
3.
Extracapsular dissection was undertaken
-
4.
The renal artery and vein were identified, ligated and divided.
-
5.
The ureter was ligated and divided close to the bladder.
-
6.
The external iliac node was then dissected from the external iliac artery
2.2. Investigations
2.2.1. Treatment
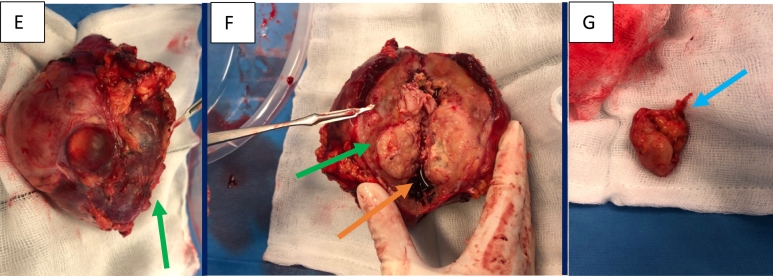
The patient underwent an open transplant nephrectomy. Care was taken to avoid injury to the functioning pancreas transplant. Intraoperative images of the transplant nephrectomy in Fig. 2E–G.
Fig. 2.
Intraoperative images of the transplant nephrectomy, cut surface and pelvic lymph node (E–G). E) Gross kidney specimen following transplant nephrectomy (green arrow), F) cut surface of kidney showing multifocal tumour (green arrow) with a bosselated mass invading into the renal pelvis, associated haemorrhage (orange arrow) is evident within collecting system G) enlarged pelvic lymph node (blue arrow).
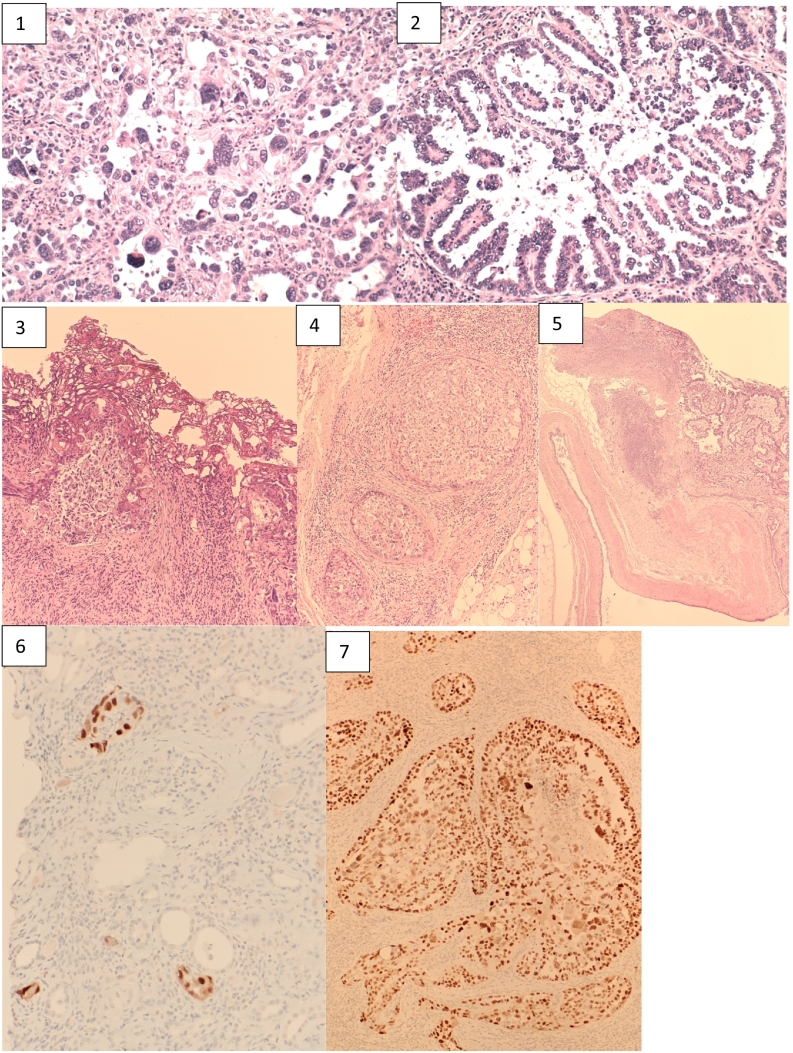
2.2.2. Histopathology (see Fig. 3)
Fig. 3.
Histopathology slides of transplant nephrectomy demonstrating Grade 4 papillary renal cell carcinoma (1–7): 1) Grade 4 carcinoma with markedly pleomorphic and hyperchromatic nuclei. Some cells display lobulated nuclei or multinucleation. 2) Area of tumour with papillary morphology. Papillary fronds are present with a core composed of loose, fibrovascular tissue. 3) RCC present at the cauterised margin of a left iliac lymph node. 4) RCC invading venous tributaries adjacent to the renal pelvis. 5) RCC invading the renal pelvis. The renal pelvis was grossly dilated by tumour, with haemorrhage also present. 6 & 7) Tumour cells are diffusely positive for SV40 immunostain, indicating the presence of BK virus. Renal tubules showing a heterogenous pattern of SV40 immunostain, consistent with polyoma virus nephropathy.
Histopathology demonstrated multifocal invasive unclassified renal cell carcinoma (RCC) grade 4*, measuring 67 mm at largest deposit. Necrosis and microvascular invasion was evident. Extrarenal extension was evident with tumour extension into the perinephric/renal sinus fat but not beyond Gerota's fascia and extension into the renal vein was seen. Two lymph nodes isolated were metastatic deposits with the largest size measuring 37 mm, extranodal extension was not seen. Immunostains show tumour cells are strongly positive for CK7. Immunostains for 34BE12 and P504S are focally positive. These features favoured a papillary subtype. The background renal parenchyma was consistent with BK virus showing prominent glomerulosclerosis, interstitial fibrosis and chronic inflammation. SV40 immunostain was positive also confirming the presence of BK virus.
*Grade (ISUP Grade) is only validated for clear cell and papillary renal cell carcinoma subtypes. Even though this lesion is unclassified it was deemed that it was close to these two subgroups and therefore the ISUP grading system was used.
2.3. Outcome and follow up
The post-operative course was uneventful, on day one post-operatively, the patient was mobilising and tolerating light diet. He was discharged home well on post-operative day 5. He has been reviewed on our outpatients clinic 4 weeks afterwards, wound had healed. He is awaiting further CT thorax, abdomen and pelvis and nuclear medicine bone scan. He is due to be seen for a medical oncology opinion regarding suitability for further treatment.
3. Discussion
A prevalence of 12 % has been reported for haematuria in renal transplant recipients [7]. Early review of renal transplant recipients who present with visible haematuria is essential to ensure prompt diagnosis and management of any underlying urological malignancy. Given that transplant recipients with cancer have been shown to have a poorer outcome than the general population due to their extensive immunosuppressive therapy the need for timely management of these patients is further amplified [4]. Urothelial malignancy is relatively low in the renal transplant cohort, with a total of <0.5 % of renal transplant recipients developing this malignancy in the literature [8,9]. However, the risk of RCC in native kidneys is 7-fold greater than the general population [5].
The development of RCC in an allograft kidney, such as in the case described here, is extremely rare. Barama et al., reported a case series of 5 patients, all with functioning renal transplants who had incidental findings of RCC <4 cm on renal ultrasound. All were treated with nephron-sparing surgical intervention [9]. Tillou et al., reported an allograft tumour incidence of 0.19 % (79 recipients of a total 41,806), with the majority being small and incidental [10]. The current treatment recommendation for solid renal masses identified in a renal transplant is the same as for that of the nontransplant population [1]. The complexity of this extracapsular retroperitoneal transplant nephrectomy was further heightened by the presence of a functioning pancreatic allograft. Given that it was a radical transplant nephrectomy due to a high grade renal carcinoma an extracapsular approach needed to be undertaken.
The genetic origin of the renal cell carcinoma cell line in this renal transplant is unclear. The native urinary tract was free from malignancy. The donor patient was young with no prior medical comorbidities and no known familial history of malignancies. Previous DNA RCC matching has been performed in the literature with recipient and donor cell lines both being reported as causative in different case reports [[11], [12], [13]].
A treatment dilemma arises in this case. In a non-immunosuppressed patient with nodal metastatic RCC disease, oncological treatment in the form of tyrosine kinase inhibitors (TKIs), checkpoint inhibitors and Mammalian target of rapamycin (mTOR) inhibitors would be considered. Vascular endothelia growth factor (VEGF) signalling pathway inhibitors such as TKIs are the first-line treatment option for patients with metastatic clear cell renal cell carcinoma in the general population. The CARMENA study reported grade 3 or 4 adverse events in 32.8 % of patients in the nephrectomy and sunitinib arm, none of whom were immunocompromised on commencement of treatment [14]. mTOR inhibitors have been shown to be an effective treatment modality in transplant recipients as both an antineoplastic therapy in the treatment of skin cancers and also as a potent immunosuppressant agent for these patients (sirolimus and everolimus) [15]. These however have significant potential side effects including reduced wound healing and reduced fertility [16]. Furthermore, it has been reported as having potential negative effects on pancreatic function which would be a deleterious consequence in this patient [17].
4. Conclusion
There are currently no guidelines on the management of allograft RCC, however its management in the literature mimics that of a non-transplanted cohort. This case report demonstrates the complexity in surgical management of RCC in a transplanted kidney in a patient with a history of simultaneous pancreas and kidney transplant. This patient will likely require further medical oncological treatment given the presence of his nodal disease.
Ethical approval
The patient provided informed, written consent for this research article. Ethical approval was granted from Beaumont Hospital Ethical Committee, Dublin, Ireland in 2021.
Funding
Nothing to declare.
Guarantor
Sarah M. Norton.
CRediT authorship contribution statement
-
1.
S. M Norton – wrote paper
-
2.
C. Norton – drew picture
-
3.
D. Hogan – edited paper
-
4.
P. Mohan – surgeon and concept of paper.
Declaration of competing interest
Nothing to declare.
References
- 1.Hoover R., Fraumeni J., Jr. Risk of cancer in renal-transplant recipients. Lancet. 1973;302(7820):55–57. doi: 10.1016/s0140-6736(73)93256-x. [DOI] [PubMed] [Google Scholar]
- 2.Moloney F.J., Comber H., O’Lorcain P., O’Kelly P., Conlon P.J., Murphy G.M. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br. J. Dermatol. 2006;154(3):498–504. doi: 10.1111/j.1365-2133.2005.07021.x. [DOI] [PubMed] [Google Scholar]
- 3.London N.J., Farmery S.M., Lodge J.P.A., Will E.J., Davidson A.M. Risk of neoplasia in renal transplant patients. Lancet. 1995;346(8972):403–406. doi: 10.1016/s0140-6736(95)92780-8. [DOI] [PubMed] [Google Scholar]
- 4.Miao Y., Everly J.J., Gross T.G., Tevar A.D., First M.R., Alloway R.R., et al. De novo cancers arising in organ transplant recipients are associated with adverse outcomes compared with the general population. Transplantation. 2009;87(9):1347–1359. doi: 10.1097/TP.0b013e3181a238f6. [DOI] [PubMed] [Google Scholar]
- 5.Hickman L.A., Sawinski D., Guzzo T., Locke J.E. Urologic malignancies in kidney transplantation. Am. J. Transplant. 2018;18(1):13–22. doi: 10.1111/ajt.14533. [DOI] [PubMed] [Google Scholar]
- 6.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surg Lond Engl. 2023;109(5):1136. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Vathsala A, Tiong HY. Haematuria in postrenal transplant patients. Biomed. Res. Int. 2015;2015. [DOI] [PMC free article] [PubMed]
- 8.Kamal M.M., Soliman S.M., Shokeir A.A., Abol-Enein H., Ghoneim M.A. Bladder carcinoma among live-donor renal transplant recipients: a single-centre experience and a review of the literature. BJU Int. 2008;101(1):30–35. doi: 10.1111/j.1464-410X.2007.07210.x. [DOI] [PubMed] [Google Scholar]
- 9.Hevia V., Gómez V., Díez Nicolás V., Álvarez S., Gómez Del Cañizo C., Galeano C., et al. Development of urologic de novo malignancies after renal transplantation. Transplant. Proc. 2014;46(1):170–175. doi: 10.1016/j.transproceed.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Barama A., St-Louis G., Nicolet V., Hadjeres R., Daloze P. Renal cell carcinoma in kidney allografts: a case series from a single center. Am. J. Transplant. 2005;5(12):3015–3018. doi: 10.1111/j.1600-6143.2005.01099.x. [DOI] [PubMed] [Google Scholar]
- 11.Tillou X., Doerfler A., Collon S., Kleinclauss F., Patard J.-J., Badet L., et al. De novo kidney graft tumors: results from a multicentric retrospective national study. Am. J. Transplant. 2012;12(12):3308–3315. doi: 10.1111/j.1600-6143.2012.04248.x. [DOI] [PubMed] [Google Scholar]
- 12.Boix R., Sanz C., Mora M., Quer A., Beyer K., Musulen E., et al. Primary renal cell carcinoma in a transplanted kidney: genetic evidence of recipient origin. Transplantation. 2009;87(7):1057–1061. doi: 10.1097/TP.0b013e31819d1e5f. [DOI] [PubMed] [Google Scholar]
- 13.Saleeb R., Faragalla H., Yousef G.M., Stewart R., Streutker C.J. Malignancies arising in allograft kidneys, with a first reported translocation RCC post-transplantation: a case series. Pathol. Res. Pract. 2015;211(8) doi: 10.1016/j.prp.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Méjean A., Ravaud A., Thezenas S., Colas S., Beauval J.-B., Bensalah K., et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N. Engl. J. Med. 2018;379(5):417–427. doi: 10.1056/NEJMoa1803675. [DOI] [PubMed] [Google Scholar]
- 15.Salgo R., Gossmann J., Schöfer H., Kachel H.G., Kuck J., Geiger H., et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am. J. Transplant. 2010;10(6):1385–1393. doi: 10.1111/j.1600-6143.2009.02997.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan B., Qazi Y., Wellen J.R. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant. Rev. 2014;28(3):126–133. doi: 10.1016/j.trre.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Barlow A.D., Nicholson M.L., Herbert T.P. Evidence for rapamycin toxicity in pancreatic β-cells and a review of the underlying molecular mechanisms. Diabetes. 2013;62(8):2674–2682. doi: 10.2337/db13-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]