Abstract
Chemokine receptors (CCRs) are important co-stimulatory molecules found on many blood cells and associated with various diseases. The expression and function of CCRs on mast cells has been quite controversial. In this study, we report for the first time that murine bone marrow-derived mast cells (BMMC) express messenger RNA and protein for CCR1. BMMC cultured in the presence of murine recombinant stem cell factor and murine IL-3 expressed CCR1 after 5–6 weeks. We also report for the first time that mBMMCCCR1+ cells endogenously express neurokinin receptor-1 and intercellular adhesion molecule-1. To examine the activity of CCR1 on these BMMC, we simultaneously stimulated two receptors: CCR1 by its ligand macrophage inflammatory protein-1α and the IgE receptor FcεRI by antigen cross-linking. We found that co-stimulation enhanced BMMC degranulation compared with FcεRI stimulation alone, as assessed by β-hexosaminidase activity (85 versus 54%, P < 0.0001) and Ca2+ influx (223 versus 183 nM, P < 0.05). We also observed significant increases in mast cell secretion of key growth factors, cytokines and chemokine mediators upon CCR1–FcεRI co-stimulation. These factors include transforming growth factor β-1, tumor necrosis factor-α and the cytokine IL-6. Taken together, our data indicate that CCR1 plays a key role in BMMC function. These findings contribute to our understanding of mechanisms for immune cell trafficking during inflammation.
Keywords: chemokines, co-stimulation, cytokines, Fc receptors, mast cells
Introduction
Allergic hypersensitivity is a major public health concern. In the United States, >54% of tested individuals have an allergic skin response to injected allergens (1), and seasonal ocular allergy affects ∼20% of Americans (2). Allergic responses have two phases: an early phase mediated by mast cells and a late phase mediated by neutrophils, T cells, eosinophils and basophils. The inflammatory vascular responses and immune cell recruitment associated with the early phase allergic response are largely caused by cytokines and chemokines, including histamine, released by mast cells. The importance of mast cells in allergic responses is well understood, but the exact mechanisms of stimulation and extent of the mast cell role in immunoregulation and acquired immunity have not been fully defined.
In addition to their role in allergic reactions, mast cells participate in inflammatory disorders, autoimmunity and innate immunity (3). Bone marrow-derived mast cells (BMMC) may have different pathways and mechanisms leading to the various morphologies and phenotypic characteristics, from immature circulating cells to mature, tissue-homing cells. These mast cell pathways and life cycles, poorly understood at this time, may be modified during inflammation. Mechanisms of mast cell inflammatory responses may involve production or response to various growth factors and chemokines. Reports of CC chemokine receptor (CCR) expression on human mast cells during inflammation (4) and mast cell regulation via growth factors and cytokines (5, 6) have initiated questions about mast cell variability in phenotype and function.
Mast cells express both CCRs and the IgE receptor FcεRI at the cell surface (4, 6). Although stimulation of FcεRI alone can activate mast cells, optimal degranulation and production of downstream chemokines and cytokines requires co-stimulatory signals (3, 7, 8). These signals may be provided by CCR1, via its ligand macrophage inflammatory protein-1α (MIP-1α) and other chemokines. CCR1 is expressed on human mast cells in several allergic disease states (7). Treatment with the CCR1 antagonist BX-471 reduced asthmatic symptoms in a murine model system (9), and we have shown that symptoms of ocular allergy are decreased in MIP-1α-deficient mice or following treatment with MIP-1α neutralizing antibodies (8). We have previously demonstrated that co-stimulation of FcεRI and CCR1 is required for optimal degranulation of CCR1-transfected rat basophilic leukemia (RBL-2H3) (10), the effects of co-stimulation on mast cells that endogenously express CCR1 remain poorly defined. BMMC are extensively used in allergic studies, but their expression of CCR1 has not been fully defined. Transcripts for CCR1 have been observed in cultured BMMC (11) and human cord blood-derived mast cells (12), but studies of protein expression have failed to detect CCR1 at the surface of mouse cells due to lack of an antibody to mouse CCR1 (13). Yanaba et al. (14) did note increased CCR1 surface expression on mouse mast cells in the Arthus reaction, but the data were not shown.
In this study, we investigated the possibility that CCR1 could be expressed on the cell surface of BMMC cultured in an appropriate combination of cytokines. We cultured the cells in the presence of IL-3 and stem cell factor (SCF) since both of these cytokines have been shown to influence mast cell proliferation, maturation and function in vivo (15). After demonstrating CCR1 expression by the cultured BMMC, we examined the effects of FcεRI-CCR1 co-stimulation on degranulation and cytokine/chemokine secretion by the mast cells.
Methods
Cell isolation and culture
Female BALB/c mice, 5–8 weeks old, were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and maintained in the animal facility of the Emory Clinic Eye Center (Atlanta, GA, USA). Bone marrow was flushed from the femurs and tibias of 9-week-old mice using a 26-gage needle and sterile PBS. Isolated cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco), 100 μg ml−1 streptomycin (Gibco), 100 U ml−1 penicillin (Gibco), 50 μM 2-mercaptoethanol (Sigma, St Louis, MO, USA), 10 ng ml−1 recombinant murine SCF and 5 ng ml−1 recombinant murine IL-3 (PeproTech, Rocky Hill, NJ, USA). The medium was changed every third day by centrifuging the cells, removing the old medium and re-suspending the cells in fresh medium. After 4 weeks of culture, >97% of the floating cells displayed a mast cell phenotype, as indicated by c-kit expression and presence of granules that stained with 0.1% toluidine blue. Further, after 8 weeks, these BMMC also acquired an adherent phenotype (NimOno BMMC).
Granulocyte lines and freshly isolated macrophages were also utilized in this study. RBL-2H3 (CRL-2256, American Type Culture Collection) and CCR1-transfected RBL-2H3 cells were cultured as described previously (10), as were HMC-1 cells, donated by J. H. Butterfield (Mayo Clinic, Rochester, MN, USA) (16). A crude macrophage suspension (>90%), isolated from the peritoneum and spleen of B6 mice, was used as a positive control for CCR1 and CCR5 expression.
Electron microscopy
Cultured cells were fixed in 1.25% glutaraldehyde at room temperature for 1 h, rinsed in 0.1 M cacodylate buffer, post-fixed in 1% osmium tetroxide for 1–2 h and washed again in 0.1 M cacodylate buffer. Cells were then en bloc stained with 2% uranyl acetate for 1 h prior to ethanol dehydration. After a final incubation in propylene oxide, the cells were embedded in resin. Sections were cut at a thickness of 1.5 μm and stained with 0.1% toluidine blue to find the area of interest. Thin sections (80–90 nm) were cut from the appropriate section of the block and incubated with 2% uranyl acetate for 30 min and then with lead citrate for 2 min. Sections were observed using a JEOL 100CXII transmission electron microscope at 60 kV.
FACS analysis of receptor expression
Surface receptor expression was analyzed by indirect immunofluorescence staining and flow cytometry. All cells were first fixed with 2% PFA. For surface expression of FcεRI, cells were labeled with anti-DNP IgE clone SPE-7 (D8406, Sigma), followed by secondary antibody GaM Fab'2 Alexa 488/Alexa 647 (Invitrogen, Carlsbad, CA, USA). CCRs, CCR1, CCR2, CCR3, CCR4, CCR5 and c-kit, were analyzed using anti-hCCR1 (MAB145 clone 53504.111), anti-hCCR2 (MAB150 clone 48607.121), anti-mCCR3 (MAB1551 clone 83103.111), anti-hCCR4 (MAB1567 clone 205410), anti-hCCR5 (MAB184 clone 45529.111) and anti-mSCF R/c-kit (AF1356), all from R&D Systems (Minneapolis, MN, USA). Murine intracellular adhesion molecule-1 (ICAM-1) was detected using mouse-specific YN1/1.7.4 antibody, produced as a rat hybridoma culture fluid (kindly donated by P. Selvaraj from Emory University, Atlanta, GA, USA), at a 1:2 dilution in PBS:0.1% bovine serum. Neurokinin-1 (NK-1) receptors were detected using a rabbit polyclonal antibody (ab8873) purchased from Abcam Inc. (Cambridge, MA, USA). Cells were incubated with primary antibody or isotype controls at 2 μg ml−1 for 1 h at 4°C, washed, blocked with the Fc blocker 2.4G2 (BD Pharmingen, San Jose, CA, USA) for 10 min and incubated with secondary antibodies. The secondary antibodies used were goat anti-rabbit F(ab’)2; goat anti-mouse IgG2b-Alexa 647 or donkey anti-goat-, donkey anti-rat- or goat anti-mouse-Alexa 488, as appropriate. The samples were processed through a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA), and data were analyzed using Flow Jo Software (Tree star, San Carlos, CA, USA).
Immunofluorescent confocal microscopy
BMMC, RBL-2H3 and RBL-CCR1 (5 × 106 cells ml−1) were fixed with 2% paraformaldehyde for 10 min on ice. The cells were stained for CCR1, -2, -3, -4 and -5, as well as for c-kit, mICAM-1 and NK-1, using the purified mAbs described above. After Fc blocking, samples were incubated with the appropriate secondary antibodies as described above. Cells were mounted with DAKO Faramount mounting medium, kept in the dark at 4°C and analyzed within 5 days. Samples were examined using the ×100 oil immersion objective of a Zeiss LSM 510 META fluorescence confocal microscope. Samples labeled with Alexa 488 were visualized using an argon laser with a 488 nm wavelength, whereas Alexa 647-labeled samples were examined using a far-red laser with a 633 nm wavelength. Z-plane optical sections were obtained <0.46 μm apart, recorded at 512 × 512 pixels and reconstructed into three-dimensional images using LSM Image Browser.
Western blot analysis of BMMC expressing CCR1
Whole-cell lysates were prepared from BMMC, RBL-2H3 and RBL-CCR1 cells using a lysis solution containing Tris buffer, nonidet P-40, dithiothreitol and protease inhibitors at pH 7.4. The lysates were centrifuged and the resulting supernatants immediately purified using protein G immunoprecipitation. Protein G beads (Sigma) were mixed with CCR1-specific antibodies (2 μg 500 μl−1 total volume) and the soluble protein lysate. The CCR1 was then eluted using a protein G column (Sigma). The eluate was mixed with Laemmli buffer at 100°C for 2 min and then the proteins were separated by PAGE and transferred to a polyvinylidene difluoride membrane. After blocking with 0.5% non-fat dry milk (Bio-Rad, Hercules, CA, USA), the membrane was probed with monoclonal anti-hCCR1 (MAB145 clone 53504.111) or with polyclonal anti-CCR1 (ab1681, Abcam, Cambridge, MA, USA) diluted 1:1000 into Tris-Tween buffered saline (TTBS)/0.1% Tween, at 4°C for 1 h. The membrane was washed three times in TTBS and then incubated with a HRP-conjugated anti-mouse or anti-rabbit secondary antibody. Immunoreactivity was visualized by applying HRP ECL substrate (Pierce, Rockford, IL, USA) and immediately exposing the membranes to x-ray film.
Reverse transcription–PCR analysis of mRNA expression
Resting and sensitized BMMC were stimulated for 30 min with 10.0 ng ml−1 dinitrylphenyl human serum albumin (DNP-HSA; Sigma) and/or various concentrations of recombinant human MIP-1α (R&D Systems) in Tyrode's buffer (DMEM containing 0.1% BSA and 10 mM HEPES). Total cellular RNA was extracted from 5 × 106 BMMC using the RNeasy kit (Qiagen, Valencia, CA, USA), following the manufacturer's instructions. The real-time PCR reaction was performed using Qiagen Quantitect SYBR Green reverse transcription (RT)–PCR kit. The PCR, performed using an Applied Biosystems 7500 real-time cycler, consisted of an initial reverse transcription step for 20 min at 50°C, denaturation for 15 min at 95°C and then 40 cycles of 94°C for 15 s, 58°C for 30 s and 72°C for 40 s. The PCR primers used for CCR1 were 5′-aaggcccagaaacaaagtct-3′ and 5′-tctgtagttgtggggtaggc-3′; CCR3, 5′-aaggacttagcaaaattcacca-3′ and 5′-acaccagggagtacagtgga-3′; CCR5, 5′-cgttccccctacaagagact-3′ and 5′-acccacaaaaccaaagatga-3′; c-kit, 5′-agaaccttctgcactcaacg-3′ and 5′-caaatcatccaggtccagag-3′; FcεRIα, 5′-gatggtcactggaaggtctg-3′ and 5′-ggtgattgttcccatagcag-3′; FcεRIβ, 5′-tgcagtgctgtttgttttgt-3′ and 5′-cgtcgtcttcggttacattc-3′ and mFcεRIγ, 5′-tgctatgggaacaatcacct-3′ and 5′-gccaatcttgcgttacattc-3′. Expression levels were normalized to that of 18srRNA (primers: 5′-atttgactcaacacgggaaa-3′ and 5′-tcgctccaccaactaagaac-3′) for each sample. All primers were chosen to recognize their respective murine complementary DNA sequences. Expression levels were compared with those of unsensitized, CCR1+ BMMC.
β-hexosaminidase assay
BMMC and RBL (1 × 106 cells ml−1) were cultured with or without 100 ng ml−1 (BMMC) or 10 ng ml−1 (RBL/RBL-CCR1) anti-DNP-IgE mAb (SPE7; Sigma) in 24-well plates overnight. For CCR1 blocking, control antagonists met-RANTES (1.25 μg ml−1, R&D systems) and BX471 (4 nM, donated by Christopher Haskell from Bayer Healthcare Pharmaceuticals) were added 5 min prior to stimulation. The cells were stimulated for 30 min with 10.0 ng ml−1 DNP-HSA and/or various concentrations of recombinant human MIP-1α in Tyrode's buffer. After stimulation, β-hexosaminidase activity was measured by incubating the supernatants with p-nitrophenyl N-acetyl-β-d-glucosamide (Sigma) in 0.1 M sodium citrate buffer (pH 4.5) for 60 min at 37°C. The reaction was stopped by adding 0.2 M glycine buffer (pH 10.5). The release of 4-p-nitrophenol was detected by measuring absorbance at 405 nm. Total β-hexosaminidase activity was determined by lysing the cells in Tyrode's buffer containing 0.1% Triton.
Determination of intracellular Ca2+ levels
RBL-CCR1 cells and BMMC (7 weeks) were harvested and re-suspended at 1 × 106 cells ml−1 in standard culture medium with or without 10–100 ng ml−1 IgE. After overnight culture at 37°C, the cells were harvested, washed with HEPES buffer saline and incubated with 1 μM Indo-1AM (Molecular Probes) in the presence of 1 μM pluronic acid (Molecular Probes) for 30 min at room temperature. The cells were than washed and re-suspended in 1.5 ml HEPES buffer saline. Intracellular Ca2+ levels were measured with and without stimulants using a fluorescence spectrometer (Digilab Hitachi, F-2500) with an excitation wavelength of 355 nm and an emission wavelength of 405 and 485 nm. CCR1 antagonists met-RANTES and BX471 were also used as described above. Maximal and minimal fluorescence levels were determined in the presence of 1% Triton X-100 and 100 mM EDTA. Intracellular Ca2+ concentrations were calculated using the formula [Ca2+] = Kd (F−F1min/F1max−F) (17).
Detection of cytokines and chemokines in cell supernatants
Cells in triplicate wells of a 24-well plate were cultured overnight in the presence or absence of 100 ng ml−1 anti-DNP IgE and then were further activated with antigen, MIP-1α or both for 30 min at 37°C. Cell supernatants were collected immediately in ice and maintained at −20°C. The murine and rat cytokines and chemokines IL-1a, IL-1b, IL-2, IL-4, IL-6, IL-10, IL-13, monocyte chemoattractant protein (MCP)-1(mJE), MIP-1α, RANTES, tumor necrosis factor-α (TNF-α) and transforming growth factor β (TGFβ)-1 were detected in the 0.3–2500 pg ml−1 range, with the analysis conducted by Pierce Biotechnology, Inc. (Woburn, MA, USA). The results were analyzed using a CCD Imaging system and SearchLight Array Analyst Software, Pierce Biotechnology, Inc.
Statistical analysis
All data are expressed as the mean ± SEM, and the comparisons between different treatments were analyzed for statistical significance by one-way analysis of variance with the Tukey's multiple comparison test using Graphpad Prism 5. Differences with P values ≤0.05 were considered to be statistically significant.
Results
CCL3/MIP-1α plays a critical role in mast cell activity in vivo (8) and enhances FcεRI-mediated mast cell functions in CCR1-expressing RBL cells in vitro (10). Since BMMC are typically used to model mast cell activity, we sought to determine if CCR1 is expressed and functional on these cells. We also wished to characterize the function of BMMC co-stimulated with antigen and the CCR1 ligand MIP-1α. Since mast cell phenotypes differ depending on their histological sites and growth environments in vivo (18), we suspected that culture conditions could play a critical role in CCR expression and functional characteristics of the BMMC. We chose to culture the BMMC in IL-3 and SCF, as these factors have been shown to stimulate mast cell production of chemokines and CCRs (11), and IL-3 has been shown to increase macrophage CCR1 expression (19).
After culturing the BMMC with IL-3 and SCF for 4 weeks, we analyzed typical histology, morphology and expression of surface markers characteristic of the mast cell phenotype. Mast cells were 98% pure after 4 weeks of culture in BMMC medium supplemented with IL-3 and SCF, as evidenced by positive toluidine blue staining (Fig. 1A). Analysis with electron microscopy demonstrated that the cells displayed typical mast cell microstructural details for the granules, nucleus, cytoplasmic content, size and cytoplasmic membrane (Fig. 1B). We next assessed cell surface expression of mast cell markers. Consistent with a mast cell phenotype, FcεRI and c-kit were expressed at the cell surface, as assessed by FACS analysis (Fig. 1C) and confocal microscopy (Fig. 1D).
Fig. 1.
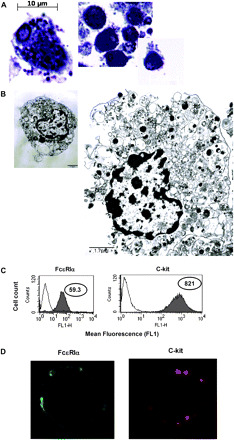
BMMC cultured in SCF and IL-3 display a mast cell phenotype. BMMC were isolated from femurs and tibias of 6-week-old BALB/c female mice and cultured with mSCF and mIL-3 for 5 weeks. (A) BMMC sections 1.5 μm thick were stained with toluidine blue and examined using a Zeiss Axioplan 2 light microscope at ×100 magnification, controlled with Zeiss Axiovision software for automatic signal acquisition. The scale bar at the bottom corner represents 20 μm. (B) BMMC sections 80–90 nm thick were examined using a JEOL 100CXII transmission electron microscope at 60 kV. The image shows typical mast cell morphology with a large eccentric nucleus surrounded with dense material concentrated around the periphery. Several dense cytoplasmic granules are also visible. The scale bar represents 1.7 μm. (C) Flow cytometry was used to examine surface expression of FcεRIα and c-kit. The control peak (‘C’) is indicated for each antibody. Data are represented in log values indicating mean fluorescent intensity (MFI). The data were analyzed using FACScan flow cytometry software. (D) Surface distribution of FcεRIα (green) and c-kit (red) was determined by immunostaining and confocal microscopy at ×100 magnification.
Interestingly, CCR1 was expressed by some of the BMMC cultured for 5 weeks, though the expression was heterogenous (Fig. 2A, left panel). CCR1 expressed on BMMC at 6 weeks of culture formed a distinct peak on the flow cytometry histogram, indicating that a large proportion of the BMMC expressed this receptor (Fig. 2A, right panel). The BMMC continued to express CCR1 over additional weeks of culture, and this phenomenon was observed consistently with three batches of BMMC isolated from different mice grown during the same time period (data not shown). The exact time point at which CCR1 expression commenced was somewhat variable, but by 6 weeks, all BMMC batches expressed CCR1. Protein expression was confirmed by confocal immunocytochemistry (Fig. 2B) and by immunoblotting with two different antibodies (Fig. 2C). RBL-2H3 used as a negative control was not fluorescent.
Fig. 2.
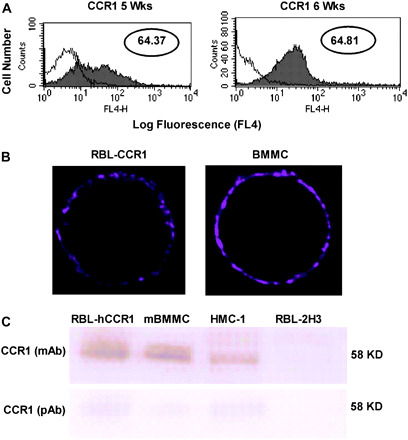
BMMC cultured in IL-3 and SCF express CCR1 protein. (A) Flow cytometry was used to examine cell surface expression of CCR1 in BMMC cultured for 5 weeks (left panel) and 6 weeks (right panel) in SCF and IL-3. The control peak (‘C’) is indicated for each antibody. Data are represented in log values indicating mean fluorescent intensity (MFI). (B) Surface distribution of CCR1 was determined for RBL-CCR1 cells (left panel) and BMMC cultured for 6 weeks in SCF and IL-3 (right panel), using immunofluorescence and confocal microscopy at ×100 magnification. (C) Western analysis was conducted using total cell lysates eluted from a protein G column and incubated with mAb and polyclonal (pAb) antibody. The two anti-CCR1 antibodies were incubated with lysates from RBL-CCR1 cells, BMMC and human mast cells (HMC-1). RBL-2H3 cells served as a negative control. With both antibodies the bands were observed at 58 kDa. The mAb is specific for human CCR1, whereas the pAb is specific for human and murine CCR1.
To further characterize the BMMC, we examined expression of a variety of molecules, related to co-stimulatory signal transduction. All batches of BMMC expressed both c-kit and FcεRI. Of the three FcεRI subunits, FcεRIγ displayed 10-fold higher expression than FcεRIα, as assessed by real-time RT–PCR, and FcεRIα expression was 2-fold higher than that of FcεRIβ (Fig. 3). No significant difference was found among subunits of α, β and γ between resting and stimulation for all conditions tested (data not shown). Of the three batches of BMMC tested, CCR2, CCR4 and CCR5 were each expressed by only one batch, with CCR5 being expressed only at very low levels in that single culture, and CCR3 was never observed (data not shown). CCR1 was therefore the predominant CCR expressed by these BMMC. Interestingly, the BMMC cultured for 6 weeks did express both ICAM-1 and NK-1, as assessed by both flow cytometry (Fig. 4A) and confocal immunofluorescence (Fig. 4B). This is the first report of these molecules being expressed by BMMC and may have impact on mast cell functions.
Fig. 3.
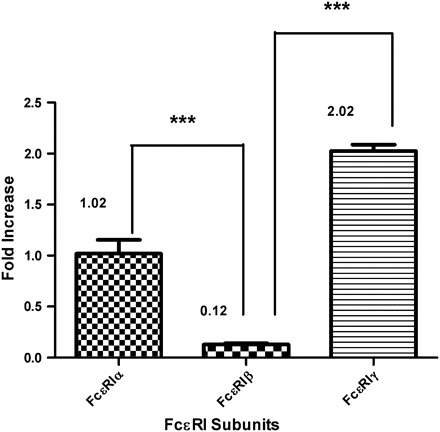
FcεRIγ is the predominant FcεRI subunit on BMMC cultured with IL-3 and SCF. Triplicate samples were collected from unstimulated cells. Real-time mRNA detection was performed using an Applied Bioscience thermocycler 7500. Expression was quantified using the Delta-delta CT calculation method and represented as fold increase. Expression levels were normalized to that of 18srRNA. For analysis of fold-change differences, everything was compared with unsensitized CCR1 expression set as 1 as shown in Fig. 5, ***P < 0.0001.
Fig. 4.
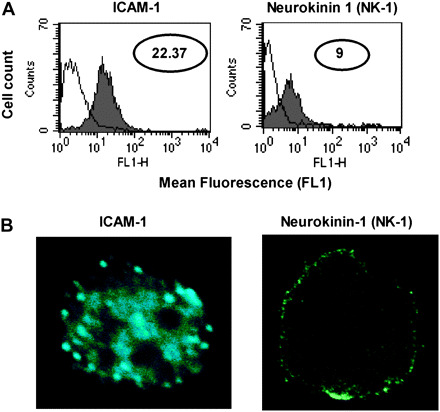
ICAM-1 and NK-1 are expressed at the surface of BMMC cultured with IL-3 and SCF. (A) Flow cytometry was used to examine cell surface expression of ICAM-1 (left panel) and NK-1 (right panel) in BMMC cultured for 6 weeks in SCF and IL-3. The control peak (‘C’) is indicated for each antibody. Data are represented in log values indicating mean fluorescent intensity (MFI). (B) Surface distribution of ICAM-1 (left panel) and NK-1 (right panel) was determined for BMMC cultured for 6 weeks in SCF and IL-3, using immunofluorescence and confocal microscopy at ×100 magnification.
Since we are particularly interested in the effects of FcεRI-CCR1 co-stimulation on mast cell activity, we examined the effects of BMMC stimulation on expression of cell surface receptors. The BMMC were first sensitized by overnight exposure to anti-DNP-IgE. Next, the cells were exposed to MIP-1α, which stimulates CCR1, and/or DNP-HSA, which cross-links and stimulates FcεRI. Levels of CCR1 messenger RNA (mRNA) were slightly, but not significantly, higher following stimulation with MIP-1α or DNP-HSA compared with resting or sensitized, unstimulated conditions (Fig. 5). CCR3 expression remained negligible for all stimulation conditions tested (Fig. 5). Levels of c-kit mRNA were not significantly different in any of the stimulation conditions (data not shown).
Fig. 5.
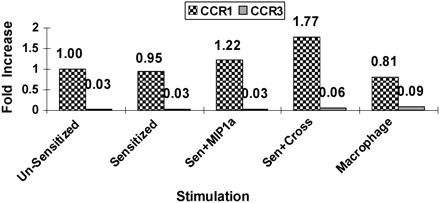
CCR1 mRNA is expressed by BMMC, and the levels vary with stimulation; CCR3 mRNA is not expressed by the BMMC under any stimulation conditions. Triplicate samples were collected from unstimulated cells or from cells stimulated for 30 min at 37°C. Real-time mRNA detection was performed using an Applied Bioscience thermocycler 7500. Expression was quantified using the Delta-delta CT calculation method and represented as fold increase. Expression levels were normalized to that of 18srRNA. For analysis of fold-change differences, CCR1 expression on unsensitized BMMC was set as one.
To examine the functionality of the CCR1 expressed on our BMMC, we examined cell degranulation and increase in Ca2+ influx following activation of FcεRI, CCR1 or both. In the histamine release assay, co-stimulation of FcεRI and CCR1 caused a significant increase in BMMC β-hexosaminidase release, to 85% activity compared with the 54% activity observed with FcεRI stimulation alone (P < 0.0001; Fig. 6B). These results were comparable to those observed for RBL-CCR1 cells (Fig. 6A). Interestingly, MIP-1α alone caused only a small amount of histamine release in BMMC (16% β-hexosaminidase activity, Fig. 6B), whereas the MIP-1α response was much stronger with RBL-CCR1 cells (61% activity; Fig. 6A). The error bars are too small to visualize.
Fig. 6.
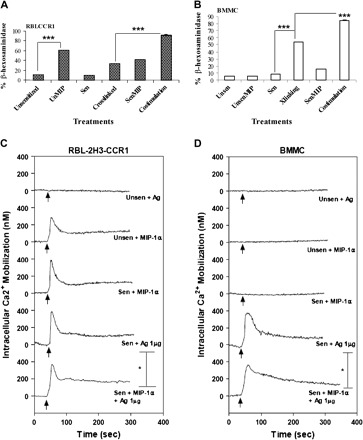
Mast cell stimulation is highest upon co-stimulation of FcεRI and CCR1 as assessed by functional assays. Percent β-hexosaminidase release was increased in (A) RBL-CCR1 cells and (B) BMMC following DNP-HSA cross-linking of FcεRI and/or stimulation of CCR1 with MIP-1α for 30 min at 37°C. Degranulation was greatest when cells were co-stimulated with both ligands. Assays were performed twice independently, with conditions tested in triplicate using 40 000 BMMC (6 weeks old) per well. Data from one typical experiment are shown, ***P < 0.0001. To assess calcium mobilization, intact RBL-CCR1 cells (C) and 7-week BMMC (D) were sensitized with 10 ng ml−1 or 100 ng ml−1 anti-DNP IgE (SPE-7), loaded with Indo 1-AM, and stimulated with antigen (10 ng ml−1 DNP-HSA) and/or MIP-1α (50 ng ml−1). The intracellular rise in calcium was immediately observed from 0 s to 5 min. Traces are representative of two independent experiments.
A similar pattern was observed for calcium influx. BMMC displayed a significant increase in calcium influx when co-stimulated (223 nM with co-stimulation versus 183 nM with FcεRI activation, P < 0.05; Fig. 6D), a degree of influx comparable to that observed for RBL-CCR1 cells (Fig. 6C). Again, MIP-1α alone caused a response with the RBL-CCR1 cells (Fig. 6C) but not the BMMC (Fig. 6D). CCR1 blocking experiments using antagonists BX471 and met-RANTES further confirmed CCR1-mediated functions as shown in Table 1.
Table 1.
Peak Ca2+ mobilization and % β-hexosaminidase release after CCR1 blocking
| Cells/treatment | Without antagonist |
CCR1 antagonist BX471 (4nM) |
CCR1/CCR5 antagonist met-RANTES (1.25 μg/ml) |
|||
| Ca+2 influx (nM) | % β-Hexosaminidase | Ca+2 influx (nM) | % β-Hexosaminidase | Ca+2 influx (nM) | % β-Hexosaminidase | |
| RBL-CCR1 | ||||||
| Unsen | NDa | 7.32 | 1.214 | 8.24 | ND | 3.23 |
| UnsenMIP | 243.21 ± 3.341 | 56.74 | 12.432 ± 3.647 | 9.62 | 3.058 ± 1.122 | 4.14 |
| Sen | ND | 6.4 | 2.67 | 6.4 | ND | 4.34 |
| SenMIP | 267.56 ± 13.321 | 44.22 | 12.0 ± 2.071 | 12.78 | 9.408 ± 3.126 | 4.66 |
| Cross-linked | 251.506 ± 4.451 | 28.67 | 127.183 ± 7.647 | 20.91 | 250.762 ± 6.671 | 17.32 |
| Co-stimulation | 316.0 ± 4.265 | 93.14 | 48.214 ± 7.013 | 53.53 | 154.0 ± 5.906 | 15.48 |
| BMMC | ||||||
| Unsen | ND | 6.13 | ND | 7.12 | ND | 4.0 |
| UnsenMIP | ND | 16.54 | ND | 6.38 | ND | 4.25 |
| Sen | ND | 8.98 | ND | 9.10 | ND | 3.43 |
| SenMIP | ND | 13.41 | ND | 8.34 | ND | 3.67 |
| Cross-linked | 142.950 ± 6.413 | 40.07 | 132.209 ± 6.383 | 40.34 | 141.693 ± 2.338 | 13.33 |
| Co-stimulation | 215.682 ± 8.277 | 83.12 | 14.124 ± 15.210 | 31.10 | 169.491 ± 7.208 | 16.16 |
RBL-CCR1 and BMMC expressing constitutive and endogenous CCR1, respectively, were sensitized overnight with IgE and challenged with MIP1α or DNP-HSA or both and also in presence of CCR1 antagonists. Peak intracellular Ca2+ mobilization was determined. Also, degranulation was determined and % β-hexosaminidase release was determined after 30 min challenge. Data are the means ± SE of three different experiments.
Not detected.
These results clearly show that CCR1 and FcεRI cooperatively mediate optimal degranulation. As a final measure of CCR1 activity in our cultured BMMC, we examined the expression of chemokines and cytokines in response to co-stimulation of CCR1 and FcεRI, compared with stimulation of FcεRI alone. IL-6, JE/MCP-1, IL-13, TNF-α and TGFβ-1 were all secreted by co-stimulated BMMC at significantly higher levels than by BMMC in which FcεRI alone was stimulated (Fig. 7B). The error bars are too small to be observed. Only the significant difference between cross-linking and co-stimulation is shown in results. A similar panel of rat specific cytokines and chemokines was observed for RBL-CCR1 cells (Fig. 7A).
Fig. 7.
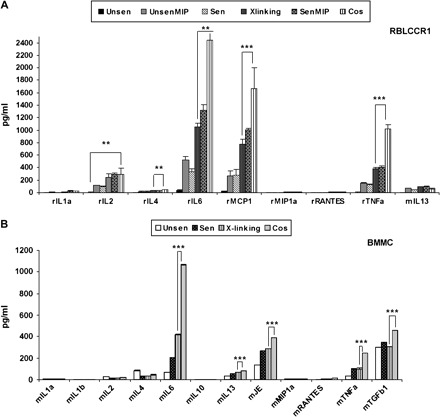
Stimulated BMMC release numerous cytokines and chemokines. Cytokine/chemokine secretion was analyzed for (A) RBL-CCR1 cells and (B) 6 weeks-cultured BMMC. Each condition was tested using 40 000 cells in triplicate wells, overnight sensitized and/or stimulated with the appropriate ligands for 30 min at 37°C. Supernatants were collected and stored/transported immediately at −20°C until analyzed. Five independent experiments were conducted. Of these, three randomly selected experiments were compared for statistical analysis. Error bars represent SEM, and statistically significant differences were identified using analysis of variance, *P < 0.05. Only the samples showing significant differences compared with co-stimulation are marked.
Discussion
Growth and function of mast cells depends on cytokines and growth factors in the external environment. If mast cells arrive at tissue sites containing SCF (e.g. conjunctiva, brain or some other parts of the nervous system) and other essential cytokines (e.g. IL-3, IL-4), the cells may attach and proliferate (20). Subsequent secretion of MCP-1, TNF-α, IL-6 and other growth factors, together with increased expression of ICAM-1 and NK-1, may regulate the trafficking of immunoregulatory cells at the site of inflammation. CCR1 function clearly contributes to mast cell activity, and BMMC cultured in IL-3 and SCF may allow better analysis of mast cell function.
Human mast cells, basophils and macrophages have been shown to express CCR1, both as mRNA transcripts and as cell surface protein (12, 21). In murine mast cells, CCR1 mRNA has been detected by RT–PCR and by in situ hybridization (11, 12, 22–24). Researchers have yet to demonstrate in vitro surface expression and function of CCR1 in murine mast cells using flow cytometry or immunoblotting. Here, we report for the first time that BMMC display surface expression of CCR1 and that this receptor is functional. Given that the previous analyses of CCR1 protein expression used different mouse strains, SCF sources, durations of culture and detecting antibodies, it is not surprising that differences in protein expression might be observed.
One limitation to the study of murine CCR1 expression is the lack of an antibody specific to mouse CCR1. For this reason, we used a mAb to human CCR1 and compared it with a rabbit polyclonal antibody known to recognize both human and mouse CCR1. Murine CCR1 was detected at 58 kDa (Fig. 1C), very similar in size to human CCR1 (25). DeVries et al. (26), conducting whole genome analysis for CCRs between species, demonstrated that the human and mouse genome sequences for CCR1 are 80% homologous. This similarity between the mouse and human sequences indicates that studies of mouse CCR1 may model function of this receptor in humans. CCR1 may participate in the pathogenesis of diseases such as multiple sclerosis, psoriasis, rheumatoid arthritis, cancer, transplant rejection and kidney disease (27), and in most of these diseases, murine models are used for research. Our discovery that appropriately cultured murine mast cells express functional CCR1 may greatly facilitate research into mast cell contributions to these diseases.
The expression of ICAM-1 and NK-1 in our BMMC cultures is particularly interesting given the roles for these proteins in mast cell activity in vitro and in vivo. ICAM-1 is expressed by human mast cells (16), and mast cell accumulation at sites of inflammation is reduced in ICAM-1-deficient mice (28). IL-4/TNF-α is known to up-regulate ICAM-1 expression (29) and may promote ICAM-1 expression on our BMMC. IL-4 secretion was detected in our cultures of unsensitized BMMC (Fig. 6B). NK-1 does not participate in adhesion but is an important communication molecule. Mast cell responses to neurite activation are directly correlated with NK-1 receptor expression in mice (30). The exact functions for this molecule on mast cells are poorly understood; expression of NK-1 by BMMC cultured in SCF and IL-3 may therefore facilitate study of nerve–mast cell communication.
Although others have observed CCR3 expression by mast cells (5, 31), we observed neither mRNA nor protein expression of this receptor in our BMMC. CCR3 expression may be suppressed by the IL-3 in the culture medium or, more likely, by TGFβ. The Tyrode's buffer used for the functional assays contains 0.1% BSA, and animal serum may contain TGFβ. Even after subtracting the value of buffer with 0.1% BSA from all final readings, significant TGFβ-1 secretion was observed under all stimulation conditions in BMMC (Fig. 4C). Sallusto et al. (32) demonstrated that TGFβ inhibits T cell expression of CCR2, -3 and -5. Interestingly, our BMMC were also negative for CCR2, -3 and -5.
We examined expression levels of the α, β and γ subunits of FcεRI, as the relative subunit levels can promote specific mast cell activities. Since the γ subunit predominated on the BMMC, FcεRIγ signaling may be actively involved in regulating cellular functions. Indeed, Yamasaki et al. (33) demonstrated that high expression of FcεRIγ during maturation and differentiation plays a role in mast cell degranulation, survival and signal transduction. Phosphorylation of FcεRγ may also amplify FcεRI cross-linking on mast cells (34). The increase in degranulation that we observe upon FcεRI cross-linking may therefore be due to higher expression of FcεRIγ.
In our functional tests for degranulation and calcium influx, MIP-1α alone did not stimulate β-hexosaminidase release or intracellular calcium influx. Cells that constitutively express CCR1, where transactivation of FcεRI is not required for generating CCR1-specific signals, may therefore have different responses to MIP-1α than do the BMMC with endogenous CCR1 expression, in which transactivation of FcεRI is required for generating CCR1-specific signals. Others, too, have found that chemokines can induce migration rather than degranulation in murine BMMC (31, 35, 36). Hartmann et al. (37) did not observe increased cytosolic calcium levels following MIP-1α stimulation of HMC-1 CCR1+ mast cells, but HMC-1 lack FcεRI signaling. When the FcεRI pathway is active, CCR1 functions as a co-stimulatory molecule and intracellular Ca2+ enhancement is observed. Interestingly, Alam et al. (38) demonstrated that the response of mouse mast cells to MIP-1α was species dependent. MIP-1α did not induce histamine release in BALB/c-derived mast cells, yet a small but significant histamine release was observed for mast cells obtained from DBA/2 mice. These results are consistent with those of our study, as our BMMC were isolated from BALB/c mice.
Also, BX471 blocking (Table 1) resulted in low calcium influx upon co-stimulation than cross-linking. This may imply the cell toxicity; however, the cell viability (trypan blue exclusion) or growth (counting cells at 10 and 30 min) assays after CCR1 blocking with antagonists showed no difference in different conditions (data not shown). Thus, it would be appropriate to hypothesize that a certain level of signaling from CCR1 is important for cell activation.
In our analysis of secreted levels for 20 cytokines and chemokines, we found that IL-6, IL-13, JE/MCP-1, TNF-α and TGFβ were secreted by BMMC at significantly higher levels after co-stimulation of FcεRI and CCR1, compared with stimulation of FcεRI alone (Fig. 7B). This is the first analysis of chemokines and cytokines secreted following FcεRI–CCR1 co-stimulation. We did not look at MIP-1α treatment alone in some tests, as this factor did not cause degranulation of BMMC when applied in the absence of antigen. Our observation of IL-6 and TNF-α up-regulation in cross-linked (FcεRI-stimulated) BMMC samples compared with unstimulated controls is consistent with previously reported results (39,40). TGFβ-1, whose secretion was enhanced following co-stimulation or cross-linking in this study, is known to contribute to allergic responses. TGFβ-1 regulates the expression of MCP-1 and plays a significant role in the effector phase of conjunctivitis by attenuating eosinophil infiltration (41,42). Further analysis of the function of these factors may help to define the role of secreted chemokines and cytokines in driving mast cell differentiation, survival and ability to recruit neutrophils and other inflammatory cells.
Funding
Dobbs Foundation.
Acknowledgments
We thank Dr Ricardo Richardson for providing RBL-hCCR1cells and Dr J. Butterfield for providing HMC-1 cells. We also thank C. A. Haskell for providing BX471. Our sincere thanks to Dr Hans E. Grossniklaus, Dr Hong Yi and Nancy L'Hernault for assisting with the electron microscopy. In addition, our sincere thanks to the Emory Winship confocal microscopy core facility members Adam Marcus, Katherine Hales and flow cytometry core director Robert Karaffa II for their support. Technical assistance with the writing of the manuscript was provided by Dr Anne Goodwin.
Glossary
Abbreviations
- BMMC
bone marrow-derived mast cells
- CCR
chemokine receptor
- DMEM
Dulbecco's modified Eagle's medium
- DNP-HAS
dinitrylphenyl human serum albumin
- ICAM-1
intracellular adhesion molecule-1
- MCP
monocyte chemoattractant protein
- MIP-1α
macrophage inflammatory protein-1α
- mRNA
messenger RNA
- NK-1
neurokinin-1
- RBL
rat basophilic leukemia
- RT
reverse transcription
- SCF
stem cell factor
- TGFβ
transforming growth factor β
- TNF-α
tumor necrosis factor-α
- TTBS
Tris-Tween buffered saline
Contributor Information
Nimita H. Fifadara, Department of Opthalmology, Dobbs Ocular Immunology Laboratories, Emory Eye Center, Emory University School of Medicine, Atlanta, GA 30322, USA
Cho Cho Aye, Department of Opthalmology, Dobbs Ocular Immunology Laboratories, Emory Eye Center, Emory University School of Medicine, Atlanta, GA 30322, USA.
Sandeep K. Raghuwanshi, JLC-Biomedical/Biotechnology Research Institute, North Carolina Central University, Durham, NC 27707, USA
Ricardo M. Richardson, JLC-Biomedical/Biotechnology Research Institute, North Carolina Central University, Durham, NC 27707, USA
Santa Jeremy Ono, Department of Opthalmology, Dobbs Ocular Immunology Laboratories, Emory Eye Center, Emory University School of Medicine, Atlanta, GA 30322, USA.
References
- 1. Arbes SJ Jr Gergen PJ Elliott L Zeldin DC Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J. Allergy Clin. Immunol. 2005;116:377. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 2. Ono SJ Abelson MB Allergic conjunctivitis: update on pathophysiology and prospects for future treatment. J. Allergy Clin. Immunol. 2005;115:118. doi: 10.1016/j.jaci.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 3. Bachelet I Levi-Schaffer F Mast cells as effector cells: a co-stimulating question. Trends Immunol. 2007;28:360. doi: 10.1016/j.it.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 4. Amin K Janson C Harvima I Venge P Nilsson G CC chemokine receptors CCR1 and CCR4 are expressed on airway mast cells in allergic asthma. J. Allergy Clin. Immunol. 2005;116:1383. doi: 10.1016/j.jaci.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 5. Ochi H Hirani WM Yuan Q Friend DS Austen KF Boyce JA T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J. Exp. Med. 1999;190:267. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ko J Yun CY Lee JS Kim DH Yuk JE Kim IS Differential regulation of CC chemokine receptors by 9-cis retinoic acid in the human mast cell line, HMC-1. Life Sci. 2006;79:1293. doi: 10.1016/j.lfs.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 7. Toda M Nakamura T Ohbayashi M et al. Mechanisms of leukocyte trafficking in allergic diseases: insights into new therapies targeting chemokines and chemokine receptors. Expert Rev. Clin. Immunol. 2007;3:351. doi: 10.1586/1744666X.3.3.351. [DOI] [PubMed] [Google Scholar]
- 8. Miyazaki D Nakamura T Toda M Cheung-Chau KW Richardson RM Ono SJ Macrophage inflammatory protein-1α as a costimulatory signal for mast cell-mediated immediate hypersensitivity reactions. J. Clin. Investig. 2005;115:434. doi: 10.1172/JCI18452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carpenter KJ Ewing JL Schuh JM et al. Therapeutic targeting of CCR1 attenuates established chronic fungal asthma in mice. Br. J. Pharmacol. 2005;145:1160. doi: 10.1038/sj.bjp.0706243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toda M Dawson M Nakamura T et al. Impact of engagement of FcεRI and CC chemokine receptor 1 on mast cell activation and motility. J. Biol. Chem. 2004;279:48443. doi: 10.1074/jbc.M408725200. [DOI] [PubMed] [Google Scholar]
- 11. Oliveira SH Lukacs NW Stem cell factor and IgE-stimulated murine mast cells produce chemokines (CCL2, CCL17, CCL22) and express chemokine receptors. Inflamm. Res. 2001;50:168. doi: 10.1007/s000110050741. [DOI] [PubMed] [Google Scholar]
- 12. Juremalm M Olsson N Nilsson G Selective CCL5/RANTES-induced mast cell migration through interactions with chemokine receptors CCR1 and CCR4. Biochem. Biophys. Res. Commun. 2002;297:480. doi: 10.1016/s0006-291x(02)02244-1. [DOI] [PubMed] [Google Scholar]
- 13. Menu E de Leenheer E de Raeve H et al. Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: a study in the 5TMM model. Clin. Exp. Metastasis. 2006;23:291. doi: 10.1007/s10585-006-9038-6. [DOI] [PubMed] [Google Scholar]
- 14. Yanaba K Mukaida N Matsushima K Murphy PM Takehara K Sato S Role of C-C chemokine receptors 1 and 5 and CCL3/macrophage inflammatory protein-1α in the cutaneous Arthus reaction: possible attenuation of their inhibitory effects by compensatory chemokine production. Eur. J. Immunol. 2004;34:3553. doi: 10.1002/eji.200425426. [DOI] [PubMed] [Google Scholar]
- 15. Wershil BK Tsai M Geissler EN Zsebo KM Galli SJ The rat c-kit ligand, stem cell factor, induces c-kit receptor-dependent mouse mast cell activation in vivo. Evidence that signaling through the c-kit receptor can induce expression of cellular function. J. Exp. Med. 1992;175:245. doi: 10.1084/jem.175.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valent P Bevec D Maurer D et al. Interleukin 4 promotes expression of mast cell ICAM-1 antigen. Proc. Natl Acad. Sci. USA. 1991;88:3339. doi: 10.1073/pnas.88.8.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cobbold PH Rink TJ Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem. J. 1987;248:313. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galli SJ Kalesnikoff J Grimbaldeston MA Piliponsky AM Williams CM Tsai M Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 2005;23:749. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 19. Jarmin DI Nibbs RJ Jamieson T de Bono JS Graham GJ Granulocyte macrophage colony-stimulating factor and interleukin-3 regulate chemokine and chemokine receptor expression in bone marrow macrophages. Exp. Hematol. 1999;27:1735. doi: 10.1016/s0301-472x(99)00115-0. [DOI] [PubMed] [Google Scholar]
- 20. Okayama Y Kawakami T Development, migration, and survival of mast cells. Immunol. Res. 2006;34:97. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brightling CE Kaur D Berger P Morgan AJ Wardlaw AJ Bradding P Differential expression of CCR3 and CXCR3 by human lung and bone marrow-derived mast cells: implications for tissue mast cell migration. J. Leukoc. Biol. 2005;77:759. doi: 10.1189/jlb.0904511. [DOI] [PubMed] [Google Scholar]
- 22. Zhao ZZ Sugerman PB Walsh LJ Savage NW Expression of RANTES and CCR1 in oral lichen planus and association with mast cell migration. J. Oral Pathol. Med. 2002;31:158. doi: 10.1034/j.1600-0714.2002.310306.x. [DOI] [PubMed] [Google Scholar]
- 23. Conti P Reale M Barbacane RC Felaco M Grilli A Theoharides TC Mast cell recruitment after subcutaneous injection of RANTES in the sole of the rat paw. Br. J. Haematol. 1998;103:798. doi: 10.1046/j.1365-2141.1998.01060.x. [DOI] [PubMed] [Google Scholar]
- 24. Eltayeb S Berg AL Lassmann H et al. Temporal expression and cellular origin of CC chemokine receptors CCR1, CCR2 and CCR5 in the central nervous system: insight into mechanisms of MOG-induced EAE. J. Neuroinflammation. 2007;4:14. doi: 10.1186/1742-2094-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Panaro MA Spinelli R Lisi S et al. Reduced expression of the chemokine receptor CCR1 in human macrophages and U-937 cells in vitro infected with Leishmania infantum. Clin. Exp. Med. 2004;3:225. doi: 10.1007/s10238-004-0029-0. [DOI] [PubMed] [Google Scholar]
- 26. DeVries ME Kelvin AA Xu L Ran L Robinson J Kelvin DJ Defining the origins and evolution of the chemokine/chemokine receptor system. J. Immunol. 2006;176:401. doi: 10.4049/jimmunol.176.1.401. [DOI] [PubMed] [Google Scholar]
- 27. Allen SJ Crown SE Handel TM Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 2007;25:787. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 28. Shimada Y Hasegawa M Kaburagi Y et al. L-selectin or ICAM-1 deficiency reduces an immediate-type hypersensitivity response by preventing mast cell recruitment in repeated elicitation of contact hypersensitivity. J. Immunol. 2003;170:4325. doi: 10.4049/jimmunol.170.8.4325. [DOI] [PubMed] [Google Scholar]
- 29. Stahl JL Cook EB Graziano FM Barney NP Human conjunctival mast cells: expression of FcεRI, c-kit, ICAM-1, and IgE. Arch. Ophthalmol. 1999;117:493. doi: 10.1001/archopht.117.4.493. [DOI] [PubMed] [Google Scholar]
- 30. Furuno T Ma D van der Kleij HP Nakanishi M Bienenstock J Bone marrow-derived mast cells in mice respond in co-culture to scorpion venom activation of superior cervical ganglion neurites according to level of expression of NK-1 receptors. Neurosci. Lett. 2004;372:185. doi: 10.1016/j.neulet.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 31. Juremalm M Nilsson G Chemokine receptor expression by mast cells. Chem. Immunol. Allergy. 2005;87:130. doi: 10.1159/000087640. [DOI] [PubMed] [Google Scholar]
- 32. Sallusto F Schaerli P Loetscher P et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998;28:2760. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 33. Yamasaki S Ishikawa E Kohno M Saito T The quantity and duration of FcRγ signals determine mast cell degranulation and survival. Blood. 2004;103:3093. doi: 10.1182/blood-2003-08-2944. [DOI] [PubMed] [Google Scholar]
- 34. Laffargue M Calvez R Finan P et al. Phosphoinositide 3-kinase γ is an essential amplifier of mast cell function. Immunity. 2002;16:441. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 35. Taub D Dastych J Inamura N et al. Bone marrow-derived murine mast cells migrate, but do not degranulate, in response to chemokines. J. Immunol. 1995;154:2393. [PubMed] [Google Scholar]
- 36. Metcalfe DD Baram D Mekori YA Mast cells. Physiol. Rev. 1997;77:1033. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 37. Hartmann K Beiglbock F Czarnetzki BM Zuberbier T Effect of CC chemokines on mediator release from human skin mast cells and basophils. Int. Arch. Allergy Immunol. 1995;108:224. doi: 10.1159/000237157. [DOI] [PubMed] [Google Scholar]
- 38. Alam R Forsythe PA Stafford S Lett-Brown MA Grant JA Macrophage inflammatory protein-1 α activates basophils and mast cells. J. Exp. Med. 1992;176:781. doi: 10.1084/jem.176.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kitaura J Xiao W Maeda-Yamamoto M Kawakami Y Lowell CA Kawakami T Early divergence of Fcε receptor I signals for receptor up-regulation and internalization from degranulation, cytokine production, and survival. J. Immunol. 2004;173:4317. doi: 10.4049/jimmunol.173.7.4317. [DOI] [PubMed] [Google Scholar]
- 40. Macleod JD Anderson DF Baddeley SM Holgate ST McGill JI Roche WR Immunolocalization of cytokines to mast cells in normal and allergic conjunctiva. Clin. Exp. Allergy. 1997;27:1328. [PubMed] [Google Scholar]
- 41. Fukushima A Sumi T Fukuda K et al. Interleukin 10 and transforming growth factor β contribute to the development of experimentally induced allergic conjunctivitis in mice during the effector phase. Br. J. Ophthalmol. 2006;90:1535. doi: 10.1136/bjo.2006.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller HR Pemberton AD Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology. 2002;105:375. doi: 10.1046/j.1365-2567.2002.01375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


