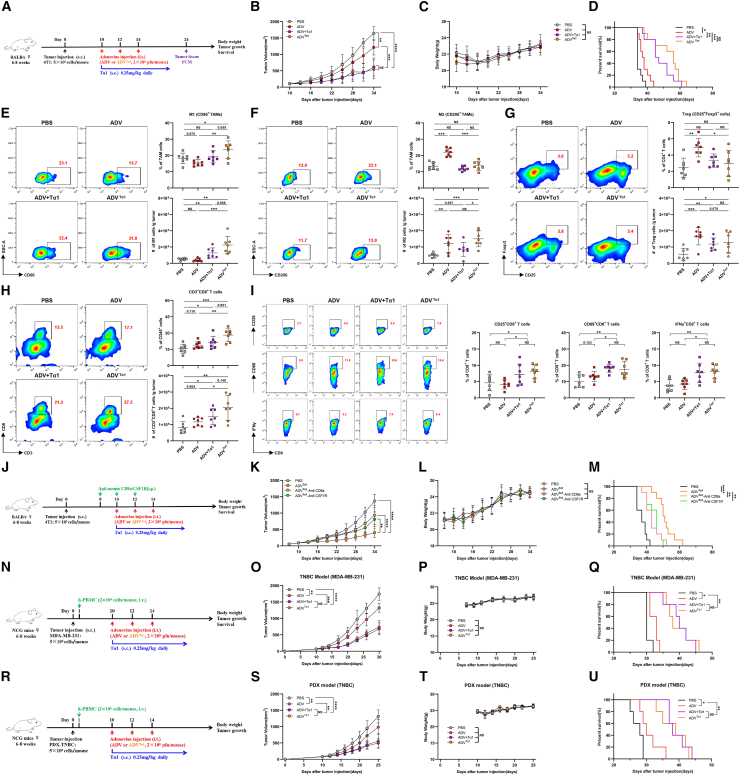
Figure 7.
The antitumor activity of ADVTα1 is mediated by macrophages and CD8+ T cells, and ADVTα1 can reprogram the TME toward a more beneficial state for antitumor immunity
(A) Experimental schematic of mice from Figures 7B–1I: 4T1 tumor-bearing WT mice were administered different immunotherapies or vehicle control (PBS) starting on day 10 when the tumor volume reached approximately 50–100 mm3. TILs from the tumor were assessed by flow cytometry on day 24; s.c., subcutaneous; i.t., intra-tumoral.
(B–D) 4T1 tumor-bearing WT mice were administered different immunotherapies or vehicle control (PBS) starting on day 10 when the tumor reached approximately 50–100 mm3 in volume (n = 10 biological replicates). (B) Tumor growth. (C) Body weight. (D) Survival.
(E–I) Representative plots (left), percentages (top), and total cells normalized to g (gram) tumor tissue (bottom) of TILs. (E) “M1-like” macrophages, (F) “M2-like” macrophages, (G) CD25+Foxp3+ Tregs, and (H) CD3+CD8+ T cells within the TME of mice. (I) Representative plots and percentages of CD25+ CD8+ T cells, CD69+ CD8+ T cells, and IFN-γ+ CD8+ T cells within the TME of mice (n = 6 biological replicates).
(J–M) 4T1 tumor-bearing mice were administered different immunotherapies and treated with intraperitoneal injections of anti-CSF1R or anti-CD8a; s.c., subcutaneous; i.t., intra-tumoral; i.p, intra-peritoneal. (J) Schematic diagram of the timeline of the antibody depletion experiment in the 4T1 model. (K) Tumor growth. (L) Body weight. (M) Survival (n = 10 biological replicates).
(N–Q) Tumor-bearing NCG mice were intravenously injected with human peripheral blood mononuclear cells on day 1, and then mice were administered different immunotherapies; s.c., subcutaneous; i.t., intra-tumoral; i.v., intravenous. (N) Schematic representation of experimental design and treatment timeline. (O) Tumor growth. (P) Body weight. (Q) Survival (n = 5 biological replicates).
(R–U) The PDX tumor cells were injected into the fourth mammary fat pads of NCG mice, and these tumor-bearing mice were intravenously injected with human peripheral blood mononuclear cells on day 1, followed by different immunotherapies starting on day 10; s.c., subcutaneous; i.t., intra-tumoral; i.v., intravenous. (R) Schematic representation of experimental design and treatment timeline. (S) Tumor growth. (T) Body weight. (U) Survival (n = 5 biological replicates).
The data are shown as the means ± SD. NS, no significant difference; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001.