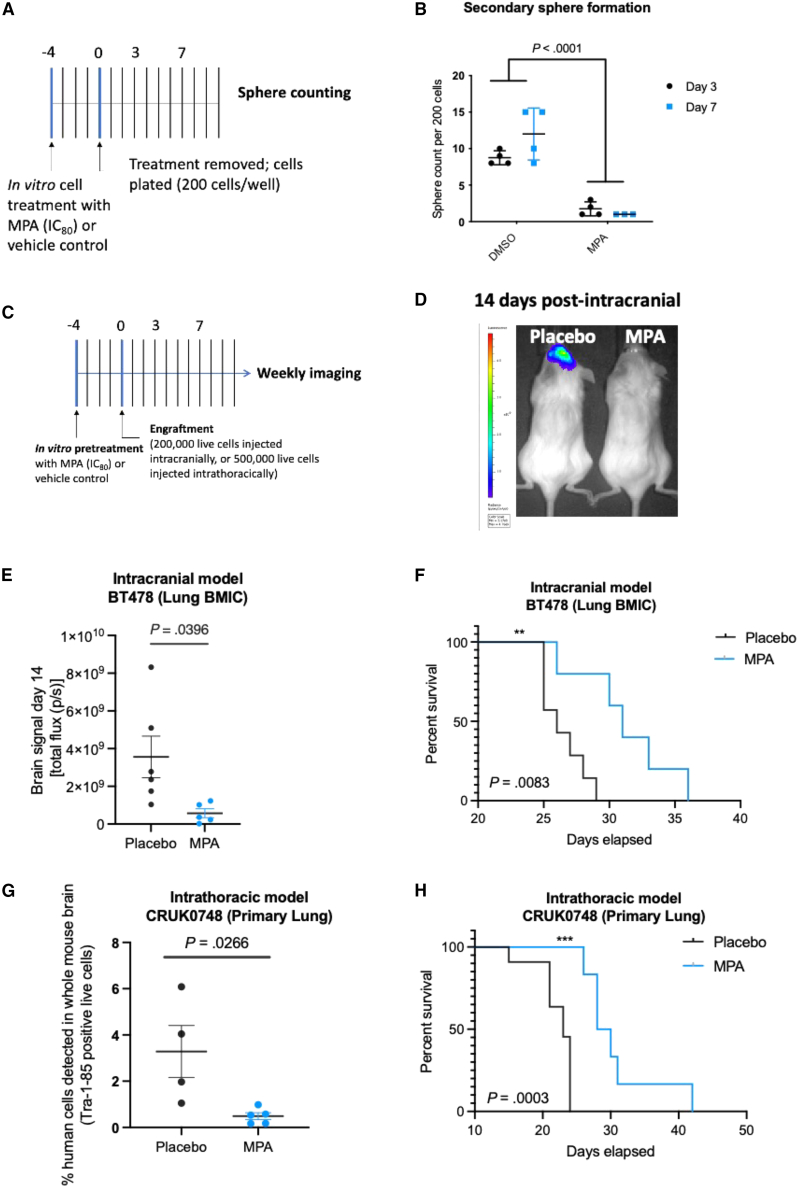
Figure 3.
Short exposure of BMICs to MPA slows BM progression in mice
(A) Schematic of clonogenic secondary sphere formation assay.
(B) Quantification of secondary tumor sphere formation by patient-derived BMICs following the removal of MPA (IC80 treatment for 72 h) or its vehicle from the culture media. Comparisons were made via a two-tailed unpaired t test and data are presented as mean ± SD from 4 to 5 technical replicates. p value is indicated.
(C) Schematic of experimental timeline. BMICs or primary lung cancer cells were pre-treated with MPA or its vehicle in vivo (due to MPA’s predicted poor BBB penetrance) for 4 days followed by either intracranial or intrathoracic engraftment into immunocompromised mice, respectively.
(D) Representative IVIS bioluminescence images of mice 14-day post injection and (E) brain tumor burden comparisons between placebo and MPA groups. IVIS, in vivo imaging system. Comparisons were made via a two-tailed unpaired t test and data are presented as mean ± SD from 4 to 5 technical replicates. p value is indicated.
(F) Kaplan-Meier survival analysis of placebo and MPA groups following intracranial engraftment of patient-derived BMICs. Comparisons were made via a log rank (Mantel-Cox) test, p value is indicated.
(G) Quantification of human cells in mouse brains by flow cytometry at the humane endpoint. Comparisons were made via a two-tailed unpaired t test and data are presented as mean ± SD from 4 to 5 technical replicates. p value is indicated.
(H) Kaplan-Meier survival analysis of vehicle and MPA groups following intrathoracic engraftment of patient-derived metastatic lung tumor cells. Comparisons were made via a log rank (Mantel-Cox) test, p value is indicated.