Scheme 2. Synthesis of ω-Functionalized HTP-Derived TFV Prodrugs Featuring Alkynyl- and Alkyl-Based Terminal Motifs.
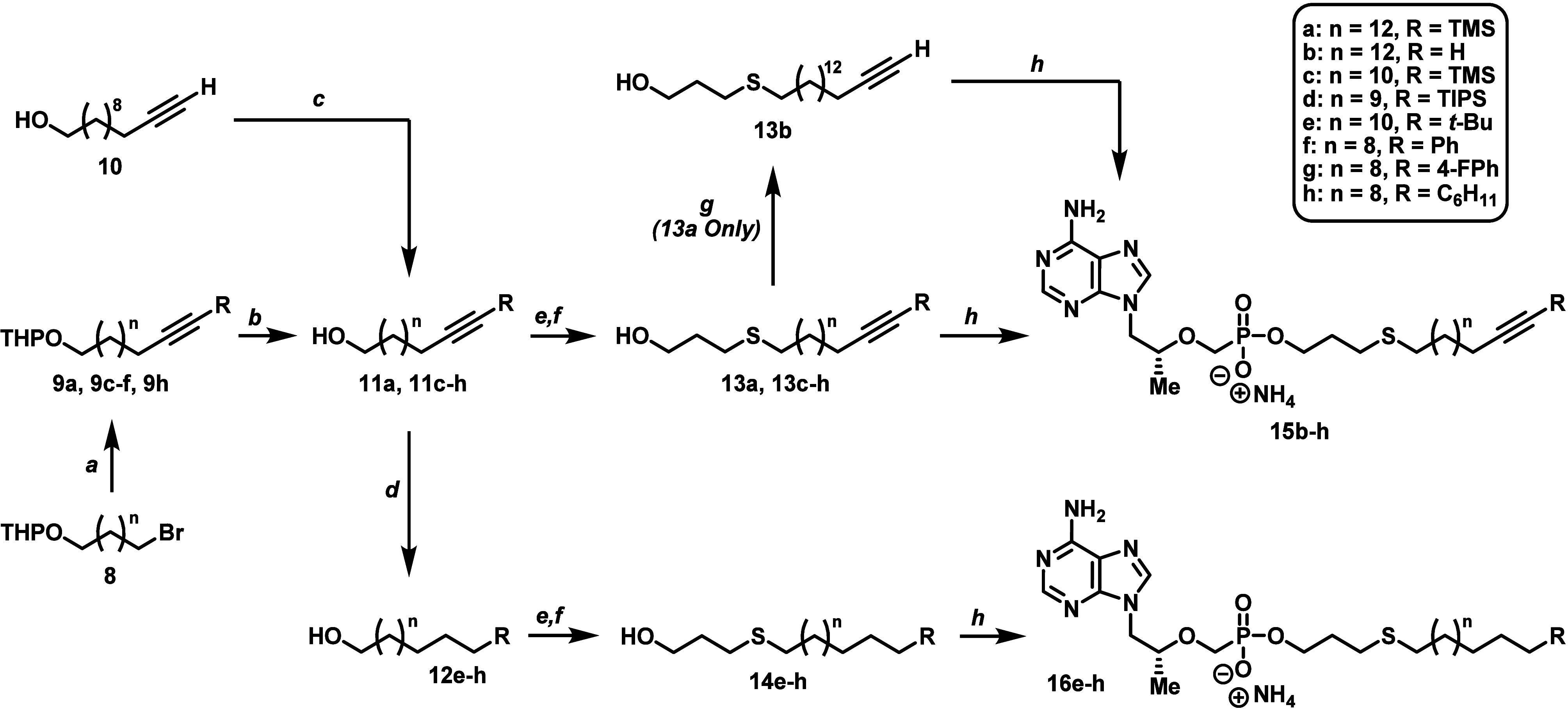
Reagents and conditions: (a) substituted acetylene, n-BuLi, HMPA, THF, −78 to −40 °C, 1 h then −40 °C to rt, 5–24 h, 84–94%; (b) p-TsOH·H2O, MeOH, rt, overnight, 69–91%; (c) 1-fluoro-4-iodobenzene, Pd(PPh3)2Cl2, CuI, Et3N, THF, rt to 70 °C, overnight, 62%; (d) 10% Pd/C, H2, MeOH or EtOAc, rt, 3–16 h, 55–83%; has MsCl, Et3N, DCM, 0 °C to rt, 45 min; (f) 3-mercaptopropanol, DBU, DMF, rt to 60–65 °C, 1.5–5 h, 68–92% over two steps; (g) TBAF, THF, rt, 45 min, 87%; (h) TFV, DCC, Et3N, DMAP, NMP, 100 °C, 18–24 h, 35–61%.
