Anatomic total shoulder arthroplasty (aTSA) is a common and reproducible procedure for treatment of primary or secondary osteoarthritis of the glenohumeral joint with good to excellent results in mid- to long-term follow-up.8,15,23 Nonetheless, glenoid polyethylene wear and component loosening remain common causes of failure after aTSA, frequently requiring revision surgery.3,24 While movement-related pain is a frequent but unspecific symptom, preoperative x-ray examinations correlate only fairly with intraoperative findings of polyethylene wear or glenoidal loosening.11 Furthermore, metal artifacts of the implanted prosthesis significantly impair magnetic resonance imaging (MRI) and largely hamper oriented diagnostics of loosening signs. Further, the presence of an additional low-grade infection can often not be excluded but strongly influences the preoperative planning of the revision surgery (one-stage vs. two-stage).
In order to accurately diagnose potential aTSA glenoid wear and minimize the exposure of radiation due to computed tomography (CT) scans, innovative, safe, and fast methods for an exact assessment and preoperative planning are required.
We therefore present the first case of an in-office, wide-awake implant related needle arthroscopy (NA) to evaluate the suspicion of glenoid wear and a low-grade infection after aTSA.
Case report
Patient
Five years after the initial treatment of primary osteoarthritis with an intact rotator cuff and a stemless aTSA with a metal back glenoid component, a 63-year-old patient presented at our outpatient department. Upon the current consultation, follow-up examinations revealed satisfactory subjective and objective results with no previous reported shoulder pain and no radiological signs of implant loosening.
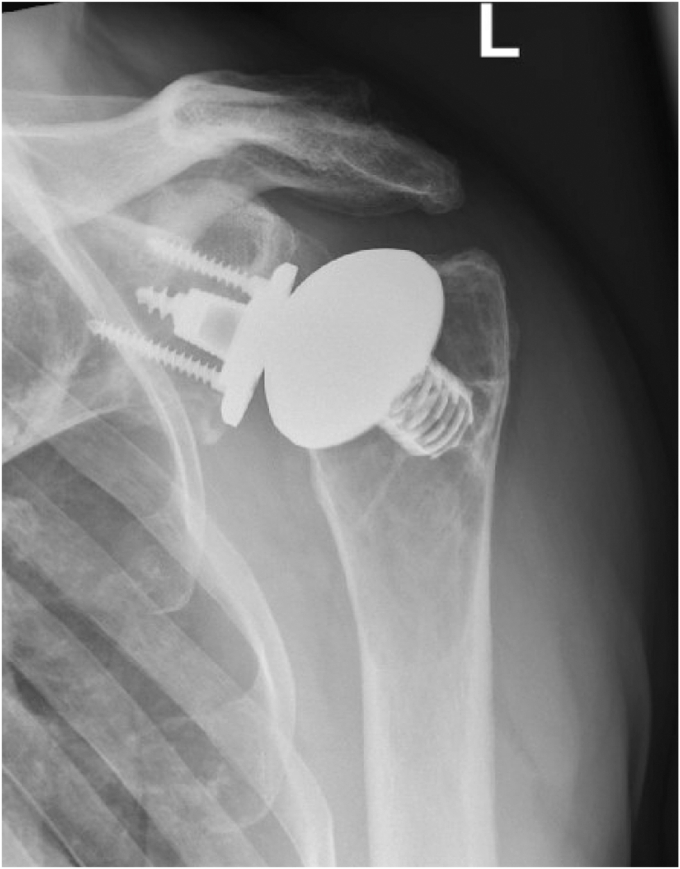
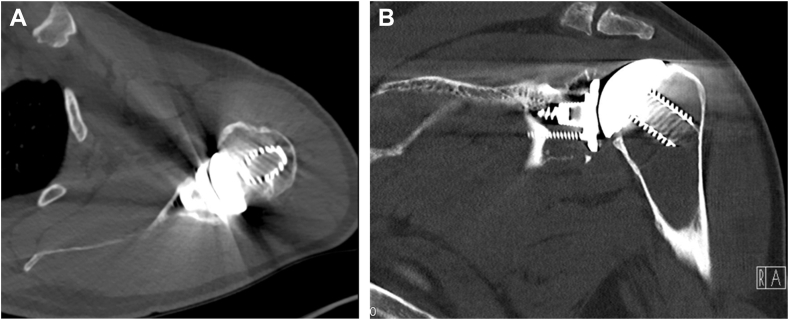
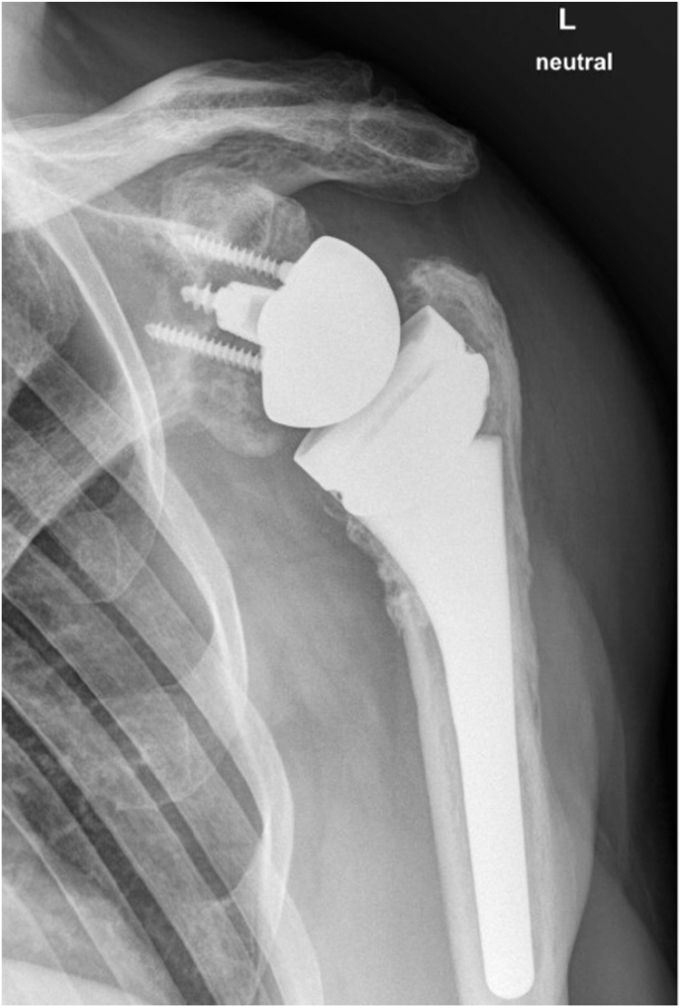
The patient reported an increasing, movement-related shoulder pain score of 9 on the visual analog scale that occurred five years after initial treatment. Range of motion was limited by pain and crepitations to 70° forward flexion, 60° abduction, and 30° external rotation. Internal rotation was limited to the buttocks. Furthermore, subscapularis tests appeared decently positive (belly press/lift off test). X-ray views (antero-posterior) and CT scans showed no clear signs of implant loosening, but there was suspicion of glenoid wear (Figs. 1 and 2).
Figure 1.
X-ray at 5-year follow-up in an antero-posterior view. Due to the movement-related pain, further x-ray views could not be performed.
Figure 2.
CT scans in (A) axillary and (B) coronal views. (A) CT scans in axillary view. (B) CT scans in coronal view. CT, computed tomography.
Due to the potential polyethylene wear or glenoid loosening and the risk of an additional low-grade infection, a diagnostic staging procedure based on a standardized NA was initiated for assessment of the glenoid implant. As well, collection of multiple biopsies were obtained to evaluate the need for either one-stage or two-stage revision surgery to a reverse shoulder arthroplasty system.
Diagnostic protocol
During the primary examination visit, the diagnostic procedure was explained to the patient, and informed consent was obtained. Blood workup with a special focus on the C-reactive protein and leukocyte count was performed after the physical examination and the standardized imaging studies. An outpatient NA was scheduled within the following days for visualization of the prosthetic status and periprosthetic soft tissue collection.
Needle arthroscopy
Preparation and placement
The patient was placed in a beach chair position in an outpatient intervention room. The screen of the needle arthroscope (Nanoscope; Arthrex, Naples, FL, USA) was placed in front of the affected side of the patient to ensure sufficient view for the surgeon and the patient. The affected arm was sterilized with antiseptics (Kodan Forte; Schülke & Mayr, Norderstedt, Germany), draped in a standardized manner, and placed in a support arm (Trimano Fortis; Arthrex, Naples, FL, USA).
Local anesthesia
The posterolateral viewing portal was prepared by infiltrating 2% lidocaine to the subcutaneous soft tissue and proceeding to the joint capsule after waiting three to five minutes to ensure a pain-free procedure.
Needle arthroscopy
Initially, a sterile syringe was used to access the joint, and 2 ml of dark turbid punctate was aspirated and saved for microbiological examination.
Further, the NA was entered into the joint by aiming in the direction of the coracoid process. Once the NA was in the joint, a standardized diagnostic arthroscopy was performed.
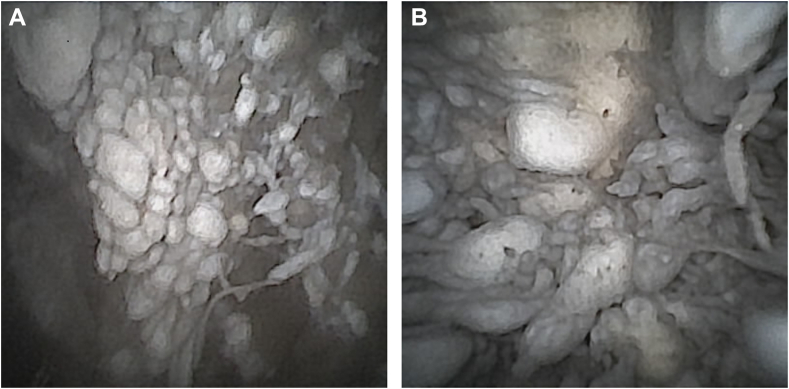
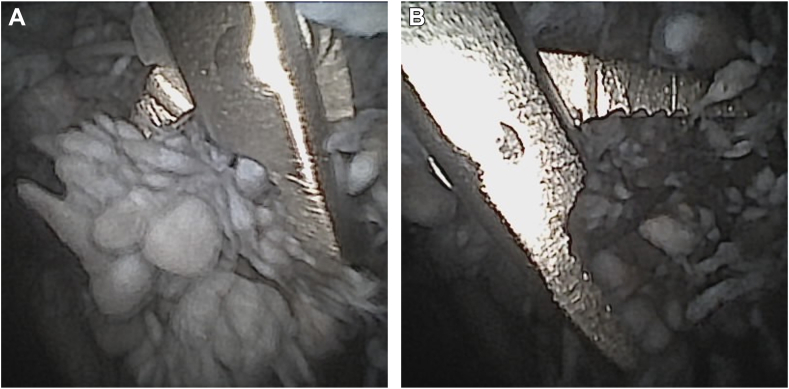
This demonstrated severe polyethylene wear of the glenoid component resulting in advanced metallosis of the soft tissue of the glenohumeral joint including the bursa and the synovia (Fig. 3). The postero-superior part of the polyethylene component was found to be entirely worn (Fig. 4). This was accompanied by consecutive grinding traces in the associated area of the humeral component (Fig. 5). The examination of the rotator cuff revealed an intact subscapularis tendon. The supraspinatus tendon appeared partially torn with articular-sided fraying. After the diagnostic arthroscopy, an antero-superior, minimally invasive cannula-based (NanoCannula 3 cm; Arthrex, Naples, FL, USA) portal was established within the rotator interval using 2% lidocaine, as it has been described for the posterior viewing portal. A probe (Nanoscope Probe; Arthrex, Naples, FL, USA) was used to confirm loosening and wear of the polyethylene component from the metal back baseplate of the aTSA. Furthermore, a nanoscopic grasper (NanoGrasper; Arthrex, Naples, FL, USA) was used to collect five soft tissue biopsies from different locations for microbiological examination (Fig. 6). The tissue samples were collected with a no-touch technique and sent to a microbiological laboratory to be processed immediately.
Figure 3.
(A) Metallosis of glenohumeral joint and the associated soft tissues. (B) Metallosis of glenohumeral joint and the associated soft tissues.
Figure 4.
Glenoid and polyethylene wear.
Figure 5.
Humeral component grinding traces.
Figure 6.
(A) Tissue collection using an NA grasper (NanoGrasper; Arthrex, Naples, FL, USA). (B) Tissue collection using an NA grasper (NanoGrasper; Arthrex, Naples, FL, USA). NA, needle arthroscopy.
After tissue collection, the NA was completed, and the incisions that did not require sutures were closed using adapting wound closure strips.
The patient was discharged 60 minutes after the intervention in good general condition.
Further, he was called 24 hours after the intervention and denied the question for major or minor complications such as vasovagal reaction, persisting pain, or allergic reaction.
Fourteen days after the intervention, the results of the soft tissue biopsies were discussed with the patient. All five biopsies showed no microbiological growth. These results combined with normal results concerning the C-reactive protein and the leukocyte count led to the decision of a single-stage revision of the aTSA to a reverse shoulder arthroplasty system that was successfully performed 7 days after the final consultation (Fig. 7).
Figure 7.
Final x-ray after conversion from aTSA to a reverse shoulder arthroplasty in an antero-posterior view. aTSA, anatomic total shoulder arthroplasty.
Discussion
The use of NA has frequently been reported in the knee, ankle, elbow, and shoulder for diagnostic but also treatment procedures.4, 5, 6, 7,9,10,12, 13, 14,16, 17, 18, 19, 20, 21, 22 This case report introduces preoperative diagnostic NA and tissue collection in cases of potential glenoid wear and/or probable low-grade infection after aTSA as a new indication for NA.
Patel et al introduced NA to the field of orthopedic surgery by presenting a diagnostic procedure to the knee for evaluation of the chondral, meniscal, and ligamental status.17 The introduction of this scope of application of NA was followed by an increased usage as a diagnostic procedure to evaluate intraarticular pathologies, which led to several cost-effective treatments.2,25 These concluded NA to be more accurate, cost-effective, and therefore economically favorable concerning diagnostics of knee pathologies like meniscal lesions.25
After promising findings of NA in the knee, Daggett et al published in 2020 a report on a standardized approach to diagnostic in-office NA of the native shoulder.6 In order to evaluate the validity of NA as a diagnostic tool for intraarticular shoulder pathologies, Wagner et al performed a prospective randomized study assessing the sensitivity and specificity of NA and MRI compared to common diagnostic arthroscopy, which was set as the gold standard.26 This study revealed similar accuracy between NA and MRI with a higher ability to rule in a diagnosis for NA (high specificities and positive predictive values) but a slightly lower ability to rule out a diagnosis (lower sensitivities and negative predictive values).26
The diagnostic usage of in-office NA in wide-awake patients with potential shoulder pathologies was further challenged and developed so that the first in-office-based intervention like the tenotomy of the long head of the biceps performed under local anesthesia was published in 2021.10,18
Furthermore, NA appears to be a viable option to perform common arthroscopic surgeries like capsulolabral repairs or rotator cuff surgery under general anesthesia in an operative setting.12,13
While prior studies and reports have constantly focused on NA in the diagnostics of native joints, this report is the first to expand the field of indications of NA to prosthetic joints with suspicion of polyethylene wear, component loosening, and/or low-grade infection.
Common arthroscopy has presented accurate results concerning the detection of periprosthetic infections by tissue collection in prior examinations but does require additional, potentially inpatient, surgery and concomitant general anesthesia as an additional general risk factor.1 In contrast, the presented diagnostic algorithm including a combination of radiological (x-ray and CT scans), clinical (physical examination and blood work), and interventional (NA) factors appears to include all pros of common arthroscopy for diagnostics of potential glenoid wear and/or low-grade infection after aTSA by overcoming multiple disadvantages of common diagnostic arthroscopy. To our knowledge, this is the first report of NA used after the implantation of a prosthetic joint.
Nevertheless, this promising pathway needs further evaluation within the next few years by higher evidence-based trials to confirm its validity and result in a common clinical diagnostic tool for primary as well as secondary joint-associated pathologies.
Conclusion
This case report introduces NA as an effective option for advanced diagnosis in painful total shoulder replacement by encompassing the majority of diagnostic features included in common shoulder arthroscopy and eliminating the necessity of general anesthesia.
Disclaimers:
Funding: This investigation was funded by Arthrex Inc. (Naples, FL, USA). The funding company was not involved in the data collection or editing of this manuscript.
Conflicts of interest: Markus Scheibel is a consultant for Stryker (Kalamazoo, MI, USA). The other authors, their immediate families, and any research foundation with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Patient consent: Obtained.
Footnotes
Institutional review board approval was not required for this case report.
References
- 1.Akgün D., Maziak N., Plachel F., Minkus M., Scheibel M., Perka C., et al. Diagnostic arthroscopy for detection of periprosthetic infection in painful shoulder arthroplasty. Arthroscopy. 2019;35:2571–2577. doi: 10.1016/j.arthro.2019.03.058. [DOI] [PubMed] [Google Scholar]
- 2.Amin N., McIntyre L., Carter T., Xerogeanes J., Voigt J. Cost-effectiveness analysis of needle arthroscopy versus magnetic resonance imaging in the diagnosis and treatment of meniscal tears of the knee. Arthroscopy. 2019;35:554–562.e13. doi: 10.1016/j.arthro.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 3.Bohsali K.I., Bois A.J., Wirth M.A. Complications of shoulder arthroplasty. J Bone Joint Surg Am. 2017;99:256–269. doi: 10.2106/jbjs.16.00935. [DOI] [PubMed] [Google Scholar]
- 4.Colasanti C.A., Mercer N.P., Garcia J.V., Kerkhoffs G.M.M.J., Kennedy J.G. In-office needle arthroscopy for the treatment of anterior ankle impingement yields high patient satisfaction with high rates of return to work and sport. Arthroscopy. 2022;38:1302–1311. doi: 10.1016/j.arthro.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Daggett M.C., Busch K., Ferretti A., Monaco E., Bruni G., Saithna A. Percutaneous anterior cruciate ligament repair with needle arthroscopy and biological augmentation. Arthrosc Tech. 2021;10:e289–e295. doi: 10.1016/j.eats.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daggett M.C., Stepanovich B., Geraghty B., Meyers A., Whetstone J., Saithna A. Office-based needle arthroscopy: a standardized diagnostic approach to the shoulder. Arthrosc Tech. 2020;9:e521–e525. doi: 10.1016/j.eats.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daggett M., Tucker T., Monaco E., Redler A., Pettegrew J., Bruni G., et al. Partial medial meniscectomy using needle arthroscopy and a standardized local anesthetic protocol. Arthrosc Tech. 2020;9:e593–e598. doi: 10.1016/j.eats.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denard P.J., Raiss P., Sowa B., Walch G. Mid- to long-term follow-up of total shoulder arthroplasty using a keeled glenoid in young adults with primary glenohumeral arthritis. J Shoulder Elbow Surg. 2013;22:894–900. doi: 10.1016/j.jse.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 9.DiBartola A.C., Rogers A., Kurzweil P., Knopp M.V., Flanigan D.C. In-office needle arthroscopy can evaluate meniscus tear repair healing as an alternative to magnetic resonance imaging. Arthrosc Sports Med Rehabil. 2021;3:e1755–e1760. doi: 10.1016/j.asmr.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauci M.-O., Monin B., Rudel A., Blasco L., Bige B., Boileau P. In-office biceps tenotomy with needle arthroscopy: a feasibility study. Arthrosc Tech. 2021;10:e1263–e1268. doi: 10.1016/j.eats.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez-Ortiz E.G., Christmas K.N., Simon P., Diaz M.A., Hess A.V., O’Briain D.E., et al. Improving preoperative planning of revision surgery after previous anatomic total shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28:S168–S174. doi: 10.1016/j.jse.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Lavender C., Lycans D., Adil S.A.S., Berdis G. Single-incision rotator cuff repair with a needle arthroscope. Arthrosc Tech. 2020;9:e419–e423. doi: 10.1016/j.eats.2019.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavender C., Lycans D., Kopiec A., Sayan A. Nanoscopic single-incision anterior labrum repair. Arthrosc Tech. 2020;9:e297–e301. doi: 10.1016/j.eats.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavender C., Patel T., Adil S., Blickenstaff B., Oliashirazi A. Incisionless knee synovectomy and biopsy with needle arthroscope and autologous tissue collector. Arthrosc Tech. 2020;9:e1259–e1262. doi: 10.1016/j.eats.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy J.C., Berglund D., Vakharia R., Tahal D.S., Mijc D., DeVito P., et al. Midterm results of anatomic total shoulder arthroplasty with a third-generation implant. J Shoulder Elbow Surg. 2019;28:698–705. doi: 10.1016/j.jse.2018.08.049. [DOI] [PubMed] [Google Scholar]
- 16.McMillan S., Saini S., Alyea E., Ford E. Office-based needle arthroscopy: a standardized diagnostic approach to the knee. Arthrosc Tech. 2017;6:e1119–e1124. doi: 10.1016/j.eats.2017.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel K.A., Hartigan D.E., Makovicka J.L., Dulle D.L., Chhabra A. Diagnostic evaluation of the knee in the office setting using small-bore needle arthroscopy. Arthrosc Tech. 2017;7:e17–e21. doi: 10.1016/j.eats.2017.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peach C., Davies R., Phillips N. Wide-awake shoulder nanoscopic long head of biceps tenotomy. Arthrosc Tech. 2021;10:e909–e912. doi: 10.1016/j.eats.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters M., Gilmer B., Kassam H.F. Diagnostic and therapeutic elbow arthroscopy using small-bore needle arthroscopy. Arthrosc Tech. 2020;9:e1703–e1708. doi: 10.1016/j.eats.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn R., Lang S.D., Gilmer B.B. Diagnostic needle arthroscopy and partial medial meniscectomy using small bore needle arthroscopy. Arthrosc Tech. 2020;9:e645–e650. doi: 10.1016/j.eats.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafi N., Lang S.D., Kassam H., Gilmer B.B. Diagnostic and therapeutic shoulder arthroscopy using a small-bore needle arthroscope. Arthrosc Tech. 2020;9:e1087–e1093. doi: 10.1016/j.eats.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stornebrink T., van Dijck R.A.H.E., Douven D., Kerkhoffs G.M.M.J. Needle arthroscopic all-inside repair of meniscal tears under local anesthesia. Arthrosc Tech. 2021;10:e2173–e2180. doi: 10.1016/j.eats.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trochia M.E., Settergren C.R. Total shoulder arthroplaty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6:495–505. doi: 10.1016/s1058-2746(97)90081-1. [DOI] [PubMed] [Google Scholar]
- 24.Vavken P., Sadoghi P., von Keudell A., Rosso C., Valderrabano V., Müller A.M. Rates of radiolucency and loosening after total shoulder arthroplasty with pegged or keeled glenoid components. J Bone Joint Surg Am. 2013;95:215–221. doi: 10.2106/jbjs.l.00286. [DOI] [PubMed] [Google Scholar]
- 25.Voigt J.D., Mosier M., Huber B. Diagnostic needle arthroscopy and the economics of improved diagnostic accuracy: a cost analysis. Appl Health Econ Health Policy. 2014;12:523–535. doi: 10.1007/s40258-014-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner E.R., Woodmass J.M., Zimmer Z.R., Welp K.M., Chang M.J., Prete A.M., et al. Needle diagnostic arthroscopy and magnetic resonance imaging of the shoulder have comparable accuracy with surgical arthroscopy: a prospective clinical trial. Arthroscopy. 2021;37:2090–2098. doi: 10.1016/j.arthro.2021.03.006. [DOI] [PubMed] [Google Scholar]