Abstract
BACKGROUND
Hepatocellular carcinoma (HCC) is a major health challenge with high incidence and poor survival rates in China. Systemic therapies, particularly tyrosine kinase inhibitors (TKIs), are the first-line treatment for advanced HCC, but resistance is common. The Rho GTPase family member Rho GTPase activating protein 12 (ARHGAP12), which regulates cell adhesion and invasion, is a potential therapeutic target for overcoming TKI resistance in HCC. However, no studies on the expression of ARHGAP12 in HCC and its role in resistance to TKIs have been reported.
AIM
To unveil the expression of ARHGAP12 in HCC, its role in TKI resistance and its potential associated pathways.
METHODS
This study used single-cell RNA sequencing (scRNA-seq) to evaluate ARHGAP12 mRNA levels and explored its mechanisms through enrichment analysis. CellChat was used to investigate focal adhesion (FA) pathway regulation. We integrated bulk RNA data (RNA-seq and microarray), immunohistochemistry and proteomics to analyze ARHGAP12 mRNA and protein levels, correlating with clinical outcomes. We assessed ARHGAP12 expression in TKI-resistant HCC, integrated conventional HCC to explore its mechanism, identified intersecting FA pathway genes with scRNA-seq data and evaluated its response to TKI and immunotherapy.
RESULTS
ARHGAP12 mRNA was found to be highly expressed in malignant hepatocytes and to regulate FA. In malignant hepatocytes in high-score FA groups, MDK-[integrin alpha 6 (ITGA6) + integrin β-1 (ITGB1)] showed specificity in ligand-receptor interactions. ARHGAP12 mRNA and protein were upregulated in bulk RNA, immunohistochemistry and proteomics, and higher expression was associated with a worse prognosis. ARHGAP12 was also found to be a TKI resistance gene that regulated the FA pathway. ITGB1 was identified as a crossover gene in the FA pathway in both scRNA-seq and bulk RNA. High expression of ARHGAP12 was associated with adverse reactions to sorafenib, cabozantinib and regorafenib, but not to immunotherapy.
CONCLUSION
ARHGAP12 expression is elevated in HCC and TKI-resistant HCC, and its regulatory role in FA may underlie the TKI-resistant phenotype.
Keywords: Hepatocellular carcinoma, Focal adhesion, Tyrosine kinase inhibitor, Rho GTPase activating protein 12, Drug resistance, Molecular mechanism, Biomarker
Core Tip: Molecular therapy has the potential to reverse tyrosine kinase inhibitors (TKIs) resistance in hepatocellular carcinoma (HCC). We combined 22861 single-cell RNA sequencing malignant cells, 5113 bulk RNA data (bulk RNA-seq and microarrays), and 562 immunohistochemistry samples to explore Rho GTPase activating protein 12 (ARHGAP12) high expression from mRNA and protein. The higher its expression, the worse the prognosis of HCC patients. HCC TKIs showed ARHGAP12 resistance. Mechanistically, we confirmed ARHGAP12 can regulate focal adhesion by combining single-cell RNA sequencing and reconfirmed it from bulk RNA of HCC and HCC TKIs. Drug sensitivity analysis showed that high expression of ARHGAP12 was associated with TKIs resistance, but not with immunotherapy.
INTRODUCTION
Hepatocellular carcinoma (HCC) has been a major challenge in the field of public health. According to statistics from 2022, liver cancer ranked the fifth in the incidence of cancer in China, with 367700 cases[1]. Among primary liver cancers, HCC is the predominant type, accounting for approximately 75%-85%[2,3]. Unfortunately, over half of HCC patients are diagnosed at the advanced stage of the disease. The five-year overall survival rate for HCC remains a concern, with only 14.1% survival rate[4-6]. Treatments for advanced HCC mostly focus on systemic therapies, with tyrosine kinase inhibitors (TKIs) being the first-line medication[7-9]. TKIs primarily inhibit tyrosine kinases involved in the vascular endothelial growth factor receptors signalling pathway in HCC, thereby reducing angiogenesis and delaying tumor recurrence[10,11]. However, in most clinical scenarios, approximately 70% of advanced HCC patients showed poor response to TKI therapy and developed primary resistance. Numerous comprehensive analyses of clinical trials indicate that the inability to identify key driver genes responsible for TKI response has been a major obstacle, which prevents HCC patients from enjoying survival benefits[12-15]. Discovery of TKI resistance genes and strategies to reverse the resistant state under TKI treatment are crucial for improving treatment outcomes in HCC patients.
As a member of the Rho GTPase family that regulates actin and cell skeleton, Rho GTPase activating protein 12 (ARHGAP12) encodes Rho GTPase-activating protein and is widely expressed in human tissues and tumor cells, potentially modulating cell invasion and adhesion[16-18]. However, aberrant expression of ARHGAP12 can drive disease progression. Reduced expression of ARHGAP12 can impact the tightness of urothelial cells in the ureteral cell line, potentially causing bladder pain syndrome[19]. Overexpression of circular ARHGAP12 can promote invasion of nasopharyngeal carcinoma cells[18]. Homozygous variants of ARHGAP12 have been identified in an early-onset colorectal cancer patient[20]. Functionally, ARHGAP12 is closely associated with adhesion. The target of ARHGAP12, Rac1, is localized in podocytes and contributes to regulating podocyte morphology, especially cell adhesion, suggesting a potential role of ARHGAP12 in cell adhesion regulation[21]. In melanoma cells, the RPEL mutant of ARHGAP12 with actin binding defects exhibits significantly increased activity in various biological assays, such as pseudopod formation, compared to the wild-type protein, and shows heightened presence at adhesion sites, facilitating intercellular adhesion[22]. TKIs are crucial in the treatment of HCC, and their resistance is associated with the formation of focal adhesions (FAs). Modulating the generation of FAs might serve as a breakthrough in reversing resistance[23]. ARHGAP12, with its adhesive function, represents a potential therapeutic target, yet there are currently no reports on the expression and role of ARHGAP12 in HCC.
This study investigates the clinical significance and potential mechanisms of ARHGAP12 in HCC. Combining bulk RNA (including RNA sequencing and microarray), immunohistochemistry (IHC), and single-cell RNA sequencing (scRNA-seq), we researchers comprehensively explore the expression of ARHGAP12 in HCC and its resistance to TKIs from both mRNA and protein perspectives. Through cellular communication and pathway enrichment analysis, we aim to elucidate the potential regulatory mechanisms of ARHGAP12 in HCC and under the action of TKIs, as well as investigate the impact of ARHGAP12 on immune therapy response and drug sensitivity to TKIs.
MATERIALS AND METHODS
Assessing the expression levels of ARHGAP12 in various cell types of HCC using scRNA-seq
To explore the value of ARHGAP12 at the scRNA-seq level, we included HCC and non-HCC in the evaluation dataset, excluded metastasis and portal vein tumor thrombus, and finally collected the dataset GSE149614 from Gene Expression Omnibus. To remove low-quality cells, cells with gene expression counts below 200 or above 8000 were excluded, unique molecular identifiers less than 200, and dead or dying cells with mitochondrial counts greater than 10% were filtered. After dimensionality reduction using the uniform manifold approximation and projection algorithm with a resolution of 0.4, we performed clustering and cell type annotation[24]. Using the inferCNV package, we identified malignant hepatocytes by comparing endothelial cells and fibroblasts as reference cells with hepatocytes[25]. Finally, we obtained endothelial cells, fibroblasts, B cells, T/natural killer cells, myeloid cells, and malignant hepatocytes to investigate the expression of ARHGAP12 in these cell types. We further analyzed the expression levels of ARHGAP12 in immune cells, stromal cells, and malignant hepatocytes using the TISCH2 database.
Potential mechanism analysis of ARHGAP12 at the scRNA-seq level
To further analyze the potential mechanisms of ARHGAP12, we identified genes positively correlated with ARHGAP12 in malignant hepatocytes from scRNA-seq data using Pearson correlation (r ≥ 0.1, P < 0.05). These genes were then subjected to enrichment analysis using the David database. Next, we scored the main FA pathway using AddModuleScore and further divided the malignant hepatocytes into high-score and low-score groups based on the median score. The “CellChat” package was also used to analyze ligand-receptor interactions within this pathway[26].
Selection and collection of gene expression data from bulk RNA resources
In order to explore whether there is homogeneity of ARHGAP12 among different ethnic groups around the world, that is, whether ARHGAP12 is at a uniform expression level in different populations. We searched and downloaded RNA-seq and gene chip datasets containing samples from HCC and non-cancerous liver tissues to identify differential expression of ARHGAP12. The inclusion criteria were as follows: (1) Samples were primarily derived from human HCC tissues; (2) HCC tissues were collected as the experimental group and non-cancerous liver tissues as the control group; and (3) There were at least 3 samples in each group. The exclusion criteria were: (1) Inadequate sample size; (2) Unextractable ARHGAP12 expression data; and (3) Samples obtained from patients with metastatic recurrent HCC. The datasets were merged according to the platform information, and abnormal data samples were removed. Inter-group correction was performed first, and then batch correction was performed using the ComBat function of sva and limma-voom (Supplementary Figure 1).
IHC staining of samples from our institution and external proteomic analysis
To explore the homogeneity of different populations and investigate the common expression level of ARHGAP12, a total of 816 tissue samples (404 HCC samples and 412 non-cancer control samples) were collected from the First Affiliated Hospital of Guangxi Medical University and Red Cross Hospital of Yulin City. IHC staining was performed according to a strict protocol. Tissues were first fixed with formalin and embedded in paraffin, and then sectioned to prepare tissue sections. Subsequently, the sections were dewaxed and hydrated with xylene, and antigen retrieval was performed in boiling ethylenediaminetetraacetic acid buffer (pH = 9.0) to restore antigenicity. After incubation with hydrogen peroxide to effectively inhibit endogenous enzyme activity, the sections were stained with a polyclonal antibody against ARHGAP12 (PA551384, Invitrogen™, dilution ratio 1:1000) and incubated at 37 °C for 30 minutes. At the end of the staining process, the slides were stained by diaminobenzidine colorimetric reaction and rinsed with phosphate buffered saline buffer between steps to remove unbound antibodies. After hematoxylin staining, the slides were dehydrated with a series of gradient ethanol solutions and finally mounted with neutral resin. The stained sections were scored under a microscope, where the brown area indicated a positive reaction and the blue area indicated a negative control. The staining results on the slides were scored as follows: 0 (no staining), 1 (pale yellow), 2 (light brown), and 3 (dark brown). The percentage of positive cells on the slides was defined using a five-point scoring system: 0 (≤ 5% of cells), 1 (6%-25% of cells), 2 (26%-50% of cells), 3 (51%-75% of cells), and 4 (≥ 76% of cells). The IHC total score was evaluated by two pathologists independently, multiplying the percentage of positive cells and staining score[27-29]. All patients provided informed consent, and this study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (No. 2020-KY-Guoji-058). Additionally, we collected proteomic data from Proteomic Data Commons for subsequent integrated analysis.
Relationship between ARHGAP12 expression and clinical parameters
Using the inclusion and exclusion criteria mentioned above, along with the availability of prognostic information, we obtained three prognostic datasets [E-TABM-36, GSE76427, and The Cancer Genome Atlas (TCGA)]. We performed Kaplan-Meier analysis to explore the relationship between ARHGAP12 expression and prognosis (Supplementary Figure 2). We further analyzed the differential expression of ARHGAP12 among various clinical and pathological parameter groups using the clinical data collected from the abovementioned IHC analysis.
Expression levels of ARHGAP12 in TKI-resistant HCC
Using the inclusion and exclusion criteria mentioned above, along with information on TKI treatment, we collected and downloaded microarray and high-throughput sequencing datasets of TKI-resistant HCC. The TKI-sensitive HCC samples served as the control group, while the TKI-resistant HCC samples were the experimental group (Supplementary Figure 3).
Potential mechanisms of ARHGAP12 in TKI resistance
First, we explored the protein-protein interaction (PPI) network of ARHGAP12 using GeneMANIA. We then integrated the resistance genes calculated by standardized mean difference (SMD) under TKI treatment with the upregulated differentially expressed genes from bulk RNA data analysis (SMD > 0, P < 0.05). We intersected these genes with the ARHGAP12 positively correlated genes obtained from Pearson correlation analysis in bulk RNA data (correlation coefficient ≥ 0.3 in at least 10 datasets, P < 0.05). The intersected genes were subjected to pathway enrichment analysis using the David database. Finally, we intersected the FA pathway genes obtained previously with genes identified in the same pathway from the scRNA-seq data.
Relationship between ARHGAP12 and potential drug treatment
TCGA data were used to analyze the effect of ARHGAP12 expression on immune escape and immunotherapy. Cellminer database and GDSC2 data were integrated to predict the relationship between ARHGAP12 expression and TKI-drug sensitivity using oncoPredict package.
Statistical analysis
To evaluate the differences in ARHGAP12 mRNA and protein expression levels in HCC and TKI-resistant HCC samples, we first used the more robust, reliable, and applicable Wilcoxon rank-sum test, and used the “pROC” package to draw receiver operating characteristic (ROC) curves to jointly analyze the ARHGAP12 expression levels of individual data. In the process of using SMD to comprehensively evaluate the ARHGAP12 expression level, when the heterogeneity was low (I2 < 50%), we used a fixed effect model to calculate the SMD; otherwise, a random effect model was used. The summary ROC (sROC) curve was drawn using STATA 16.0, and the larger the area under the curve (AUC), the higher the ARHGAP12 expression level. The Egger’s and Begg’s tests were used to assess publication bias, and P > 0.05 indicated no publication bias. Except for the Egger’s and Begg’s tests, P < 0.05 was considered statistically significant. Figure 1 showed the overall framework design of this research.
Figure 1.
Research overflow of this study. ARHGAP12: Rho GTPase activating protein 12; HCC: Hepatocellular carcinoma; TKI: Tyrosine kinase inhibitor.
RESULTS
The high expression of ARHGAP12 in malignant hepatocytes
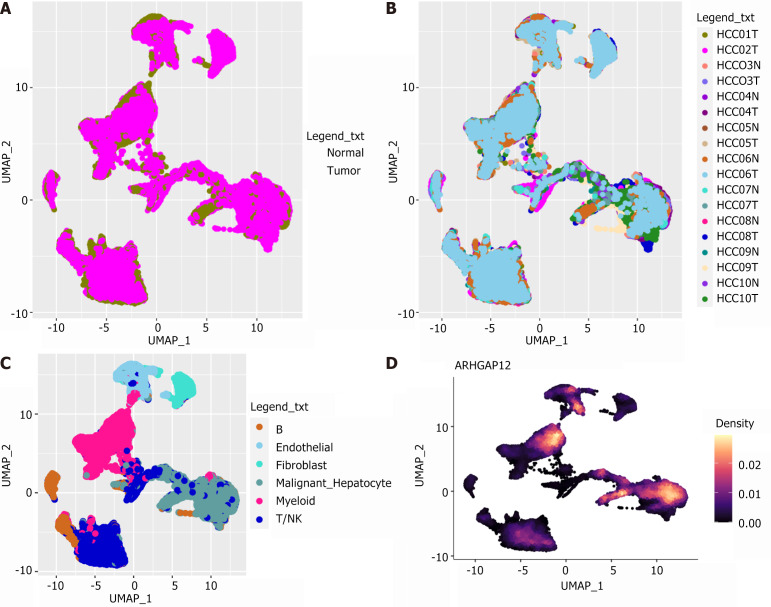
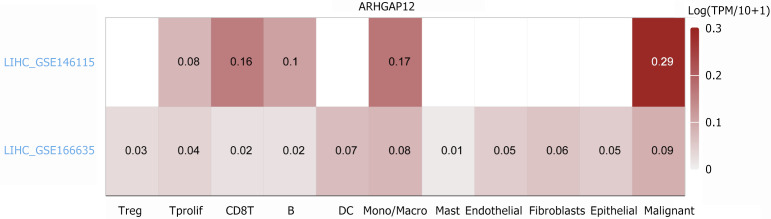
With the help of the inferCNV package, malignant hepatocytes were identified from hepatocytes based on epithelial cells and fibroblasts. The results showed that ARHGAP12 was significantly upregulated in 15990 malignant hepatocytes (Figure 2). Data from the TISCH2 database revealed high expression of ARHGAP12 in 4500 malignant cells from LIHC_GSE166635 and 2371 malignant cells from LIHC_GSE146115 (Figure 3).
Figure 2.
Single-cell RNA sequencing data analysis of 18 primary hepatocellular carcinoma and non-hepatocellular carcinoma tissue samples in GSE149614. A: Cell distribution of hepatocellular carcinoma and non-hepatocellular carcinoma groups in the samples; B: Distribution of included samples. Different colors represent different tissue samples; C: Main cell types; D: Rho GTPase activating protein 12 expression distribution. ARHGAP12: Rho GTPase activating protein 12; HCC: Hepatocellular carcinoma; UMAP: Uniform manifold approximation and projection.
Figure 3.
Expression levels of Rho GTPase activating protein 12 in various cell types in the TISCH2 database. ARHGAP12: Rho GTPase activating protein 12.
The potential molecular mechanisms of ARHGAP12 at the scRNA-seq level
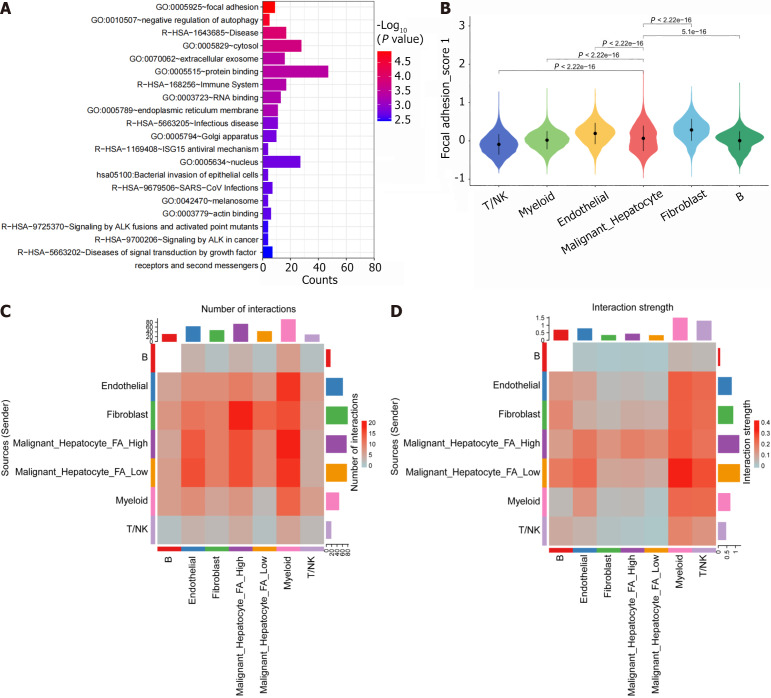
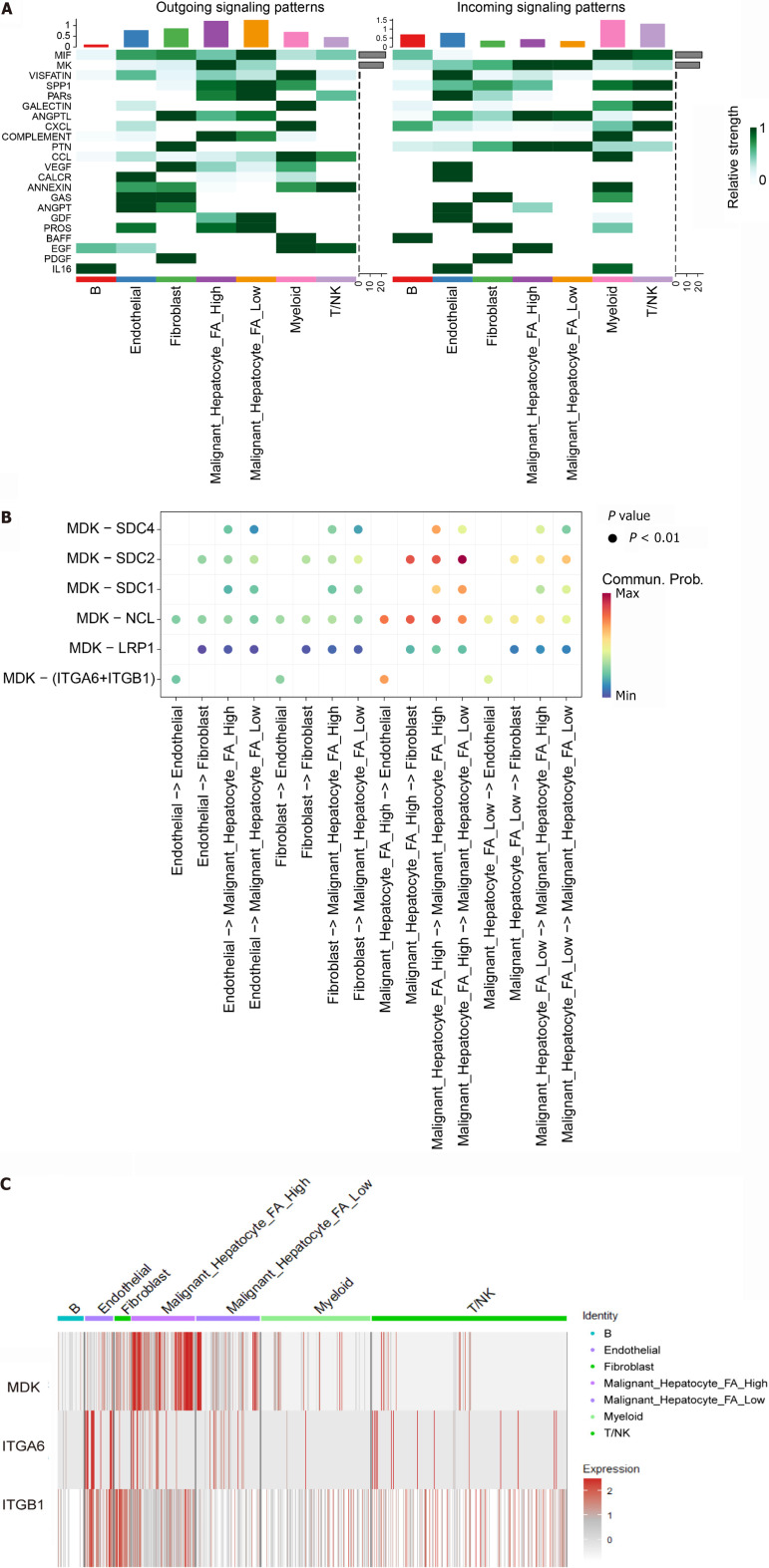
To explore the potential mechanisms of ARHGAP12, we performed enrichment analysis on ARHGAP12-correlated genes (r ≥ 0.1, P < 0.05) in malignant hepatocytes. The results revealed that the FA pathway ranked first in terms of enrichment (Figure 4A). Within the FA pathway score, fibroblast cells, endothelial cells, and malignant hepatocytes ranked as the top three cell types, with higher scores indicating higher pathway gene expression within the gene set (Figure 4B). Cellular communication analysis revealed a greater number of interaction networks between malignant hepatocytes with high FA pathway scores and fibroblast cells, while malignant hepatocytes with low FA pathway scores exhibited stronger interactions with myeloid cells (Figure 4C and D).
Figure 4.
Investigation of the potential mechanisms of Rho GTPase activating protein 12 in the focal adhesion pathway. A: Enrichment analysis; B: Focal adhesion pathway scores in various cell types; C: Number of interactions in various cell types; D: Strength of interactions in various cell types. FA: Focal adhesion.
Additionally, to further investigate the potential mechanisms of the FA pathway, we identified the interaction between ligands and receptors within the pathway. In the high FA pathway score group of malignant hepatocytes, the strongest incoming and outgoing actions were observed in the MK signaling transduction (Figure 5A). In the ligand-receptor analysis targeting MK, the signal transmission from MDK-(ITGA6 + ITGB1) to endothelial cells was found to be stronger in the high FA pathway score group of malignant hepatocytes (Figure 5B). MDK exhibited the highest expression in the high FA pathway score group of malignant hepatocytes in heatmap (Figure 5C).
Figure 5.
Analysis of the MK communication network. A: Heatmap of outgoing and incoming signal patterns; B: Ligand-receptor network of MK signaling; C: Heatmap plot of MDK-(integrin alpha 6 + integrin β-1) expression. ITGA6: Integrin alpha 6; ITGB1: Integrin β-1; FA: Focal adhesion.
Upregulation of ARHGAP12 mRNA in bulk RNA data
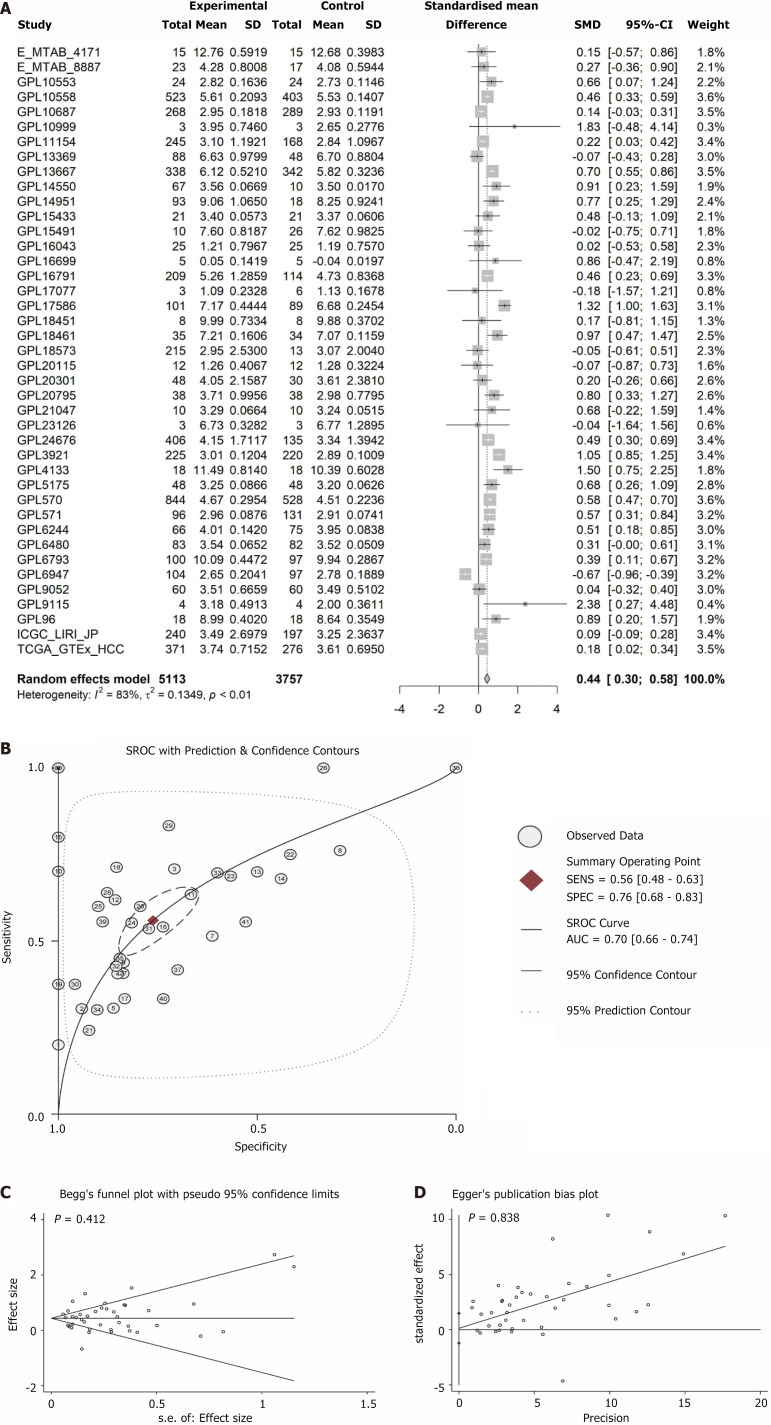
According to the Wilcoxon rank-sum test, ARHGAP12 mRNA showed significant overexpression across 10 platforms (P < 0.05, AUC ≥ 0.7, Supplementary Figures 4, 5, 6 and 7). In the random-effects model, comprehensive overexpression of ARHGAP12 mRNA was observed in 5113 HCC samples [SMD = 0.44, 95% confidence interval (CI): 0.30-0.58, Figure 6A]. Furthermore, the sROC analysis confirmed the high expression of ARHGAP12 mRNA (AUC = 0.70, Figure 6B). Egger’s and Begg’s tests indicated the absence of publication bias (P = 0.412 and 0.838) (Figure 6C and D).
Figure 6.
Integrated analysis on Rho GTPase activating protein 12 expression in hepatocellular carcinoma. A: Forest plot; B: Summary receiver operating characteristic curve; C: Egger’s; D: Begg’s. SMD: Standardized mean difference; CI: Confidence interval; sROC: Summary receiver operating characteristic; AUC: Area under the receiver operating characteristic curve.
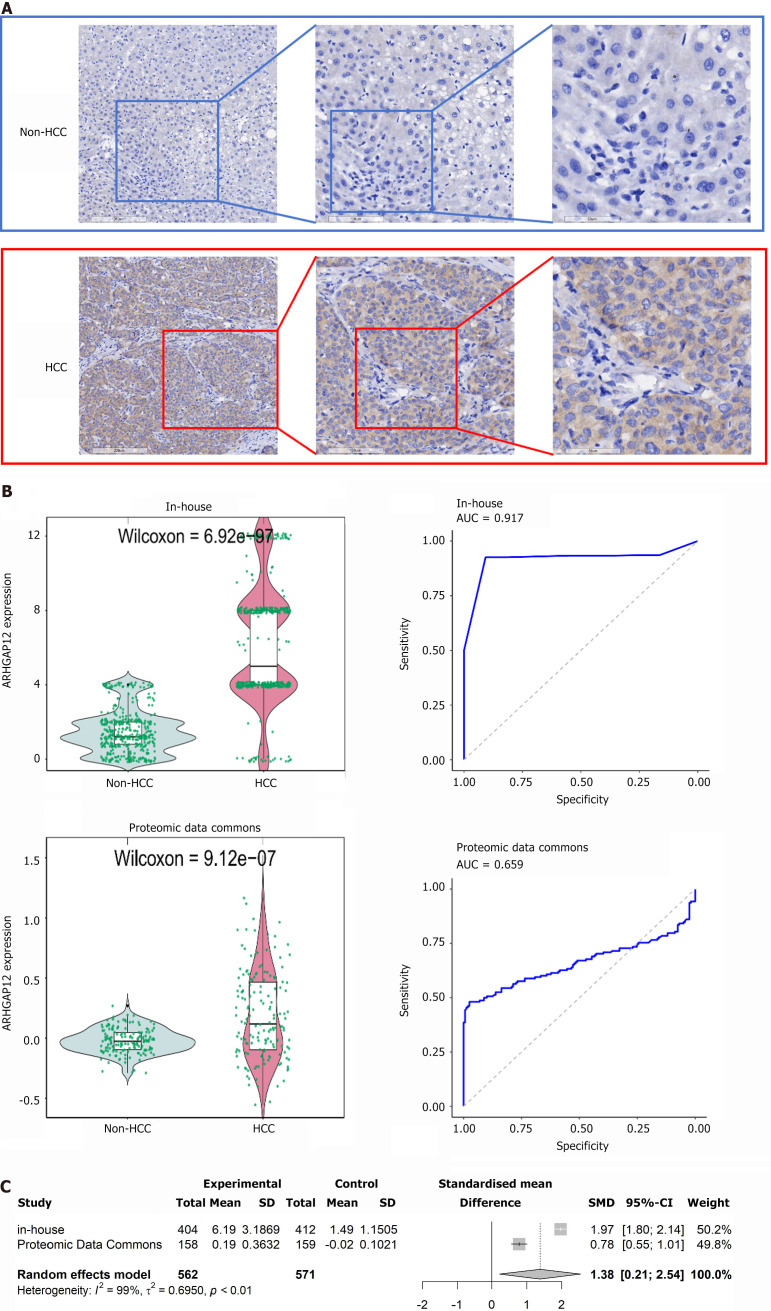
Upregulation of ARHGAP12 protein in HCC tissues
Immunohistochemical data indicated intense staining of ARHGAP12 antibody in HCC tissues (Figure 7A). Violin plots and ROC curves demonstrated the high expression of ARHGAP12 protein (Figure 7B). Combining in-house immunohistochemical data with proteomic data further confirmed the upregulation of ARHGAP12 protein in 562 HCC samples (SMD = 1.38, 95%CI: 0.21-2.54, Figure 7C).
Figure 7.
Expression of Rho GTPase activating protein 12 protein in hepatocellular carcinoma tissues. A: Immunohistochemistry staining of control and hepatocellular carcinoma samples; B: Violin plots and receiver operating characteristic curves; C: Comprehensive expression levels of Rho GTPase activating protein 12 protein. ARHGAP12: Rho GTPase activating protein 12; HCC: Hepatocellular carcinoma; SMD: Standardized mean difference; CI: Confidence interval; AUC: Area under the receiver operating characteristic curve.
Relationship of high ARHGAP12 expression in HCC with clinical pathological progression
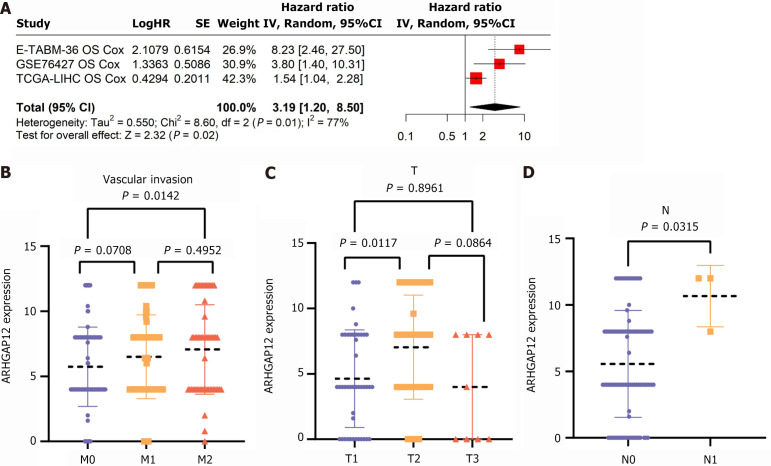
To further investigate the relationship between ARHGAP12 expression and clinical significance, integrated prognosis analysis indicated that higher ARHGAP12 expression correlated with poorer patient prognosis (Figure 8A). The scatter plots showed that ARHGAP12 expression was higher in stage M2 compared to stage M0 in vascular invasion, higher in stage T2 compared to stage T1, and higher in stage N1 compared to stage N0 in lymph node staging (Figure 8B, C and D). Table 1 presented the statistical data regarding ARHGAP12 expression in clinical samples in relation to age, gender, pathological grading, tumor necrosis, satellite nodules, vascular invasion, and tumor-node-metastasis stages, with significant differences observed in gender, vascular invasion, and TN staging (P < 0.05).
Figure 8.
Relationship of Rho GTPase activating protein 12 with clinical pathological parameters in hepatocellular carcinoma. A: Comprehensive prognostic levels of Rho GTPase activating protein 12; B: Vascular invasion; C: T stage; D: N stage. ARHGAP12: Rho GTPase activating protein 12; CI: Confidence interval; OS: Overall survival; HR: Hazard ratio.
Table 1.
Expression of Rho GTPase activating protein 12 in different groups based on clinical pathological features
|
Clinicopathological features
|
ARHGAP12 protein expression
|
t (t-test) or F (ANOVA)
|
P value
|
||
|
Number
|
mean
|
SD
|
|||
| Age | 1.6400 | 0.1017 | |||
| > 60 | 101 | 5.743 | 3.504 | ||
| ≤ 60 | 303 | 6.342 | 3.065 | ||
| Gender | 2.1130 | 0.0352 | |||
| Male | 339 | 6.046 | 3.172 | ||
| Female | 65 | 6.954 | 3.179 | ||
| Pathology grade | 1.4470 | 0.2366 | |||
| Grade I-II | 203 | 6.043 | 3.347 | ||
| Grade II-III | 85 | 5.995 | 3.099 | ||
| Grade III-IV | 113 | 6.630 | 2.960 | ||
| Tumor necrosis | 1.5980 | 0.1112 | |||
| Yes | 178 | 6.279 | 2.931 | ||
| No | 80 | 5.570 | 3.989 | ||
| Satellite nodule | 0.7686 | 0.2944 | |||
| Yes | 81 | 6.079 | 3.145 | ||
| No | 287 | 6.199 | 3.275 | ||
| Vascular invasion | 4.9580 | 0.0075 | |||
| M0 | 199 | 5.733 | 3.048 | ||
| M1 | 142 | 6.501 | 3.217 | ||
| M2 | 58 | 7.062 | 3.438 | ||
| TNM stages | |||||
| T | 5.1990 | 0.0072 | |||
| T1 | 45 | 4.631 | 3.734 | ||
| T2 | 44 | 7.063 | 3.982 | ||
| T3 | 9 | 4.000 | 4.000 | ||
| N | 2.1830 | 0.0315 | |||
| N0 | 96 | 5.563 | 4.016 | ||
| N1 | 3 | 10.670 | 2.309 | ||
| M | 1.5650 | 0.1208 | |||
| M0 | 98 | 5.653 | 4.035 | ||
| M1 | 1 | 12.000 | 0.000 | ||
| HBV | 0.3460 | 0.7296 | |||
| HBV-positive | 182 | 6.332 | 3.626 | ||
| HBV-negative | 62 | 6.155 | 3.002 | ||
Microvascular invasion grading: M0 level: Microvascular invasion not found; M1 grade: ≤ 5 microvascular invasions and occur in the area of proximal adjacent liver tissue (≤ 1 cm); M2 grade: > 5 microvascular invasions, or microvascular invasions that occur in the area of liver tissue distally adjacent to the cancer (> 1 cm). ARHGAP12: Rho GTPase activating protein 12; TNM: Tumor-node-metastasis; HBV: Hepatitis B virus.
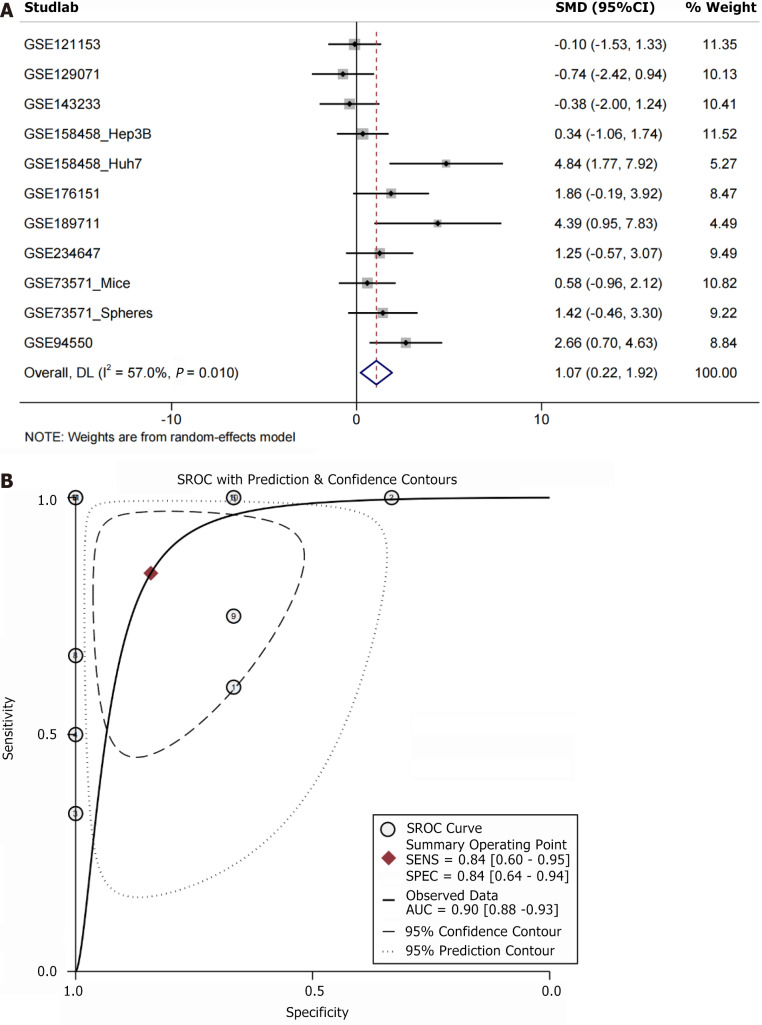
Upregulation of ARHGAP12 mRNA under the TKIs
Under the treatment of TKIs, two datasets demonstrated high expression of ARHGAP12 mRNA (P < 0.05, AUC ≥ 0.7, Supplementary Figure 8). Integrated analysis revealed a comprehensive upregulation of ARHGAP12 mRNA in 41 TKI-resistant HCC samples (SMD = 1.07, 95%CI: 0.22-1.92, Figure 9A). Likewise, the sROC curve demonstrated the high expression of ARHGAP12 mRNA in TKI resistance (AUC = 0.90, Figure 9B).
Figure 9.
High expression of Rho GTPase activating protein 12 under tyrosine kinase inhibitors treatment. A: Forest plot showing elevated expression of Rho GTPase activating protein 12; B: Summary receiver operating characteristic curve plot. SMD: Standardized mean difference; CI: Confidence interval; sROC: Summary receiver operating characteristic; AUC: Area under the receiver operating characteristic curve.
Potential regulatory mechanisms of ARHGAP12 in TKI-resistant HCC
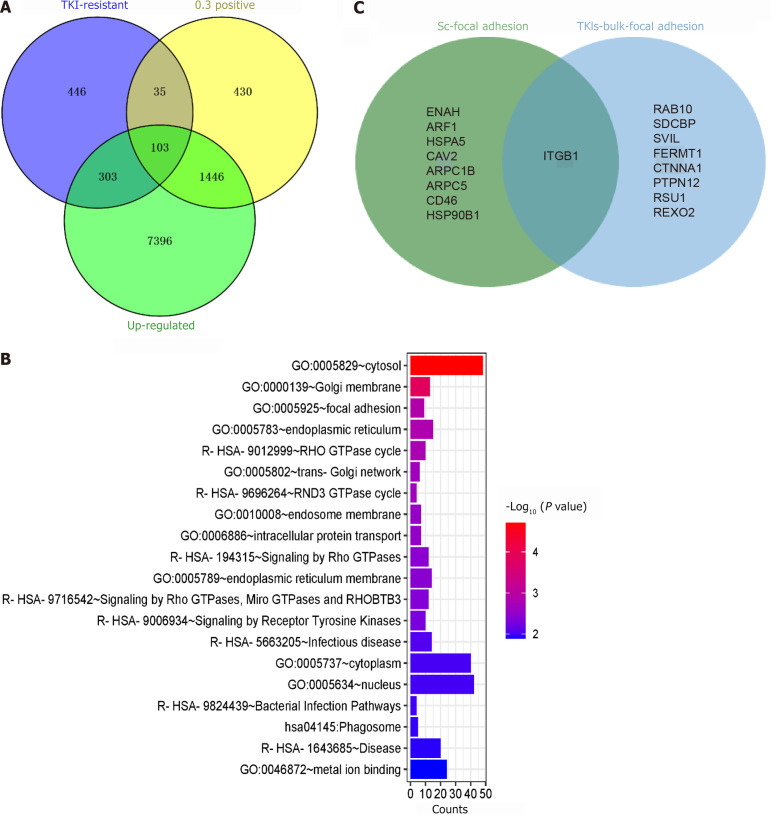
To further explore the potential functions of ARHGAP12 and its regulatory mechanisms in TKI treatment, we employed PPI analysis to investigate the potential interacting factors of ARHGAP12. We found that ARHGAP12 was involved in endocytosis and regulates cellular morphological changes (Supplementary Figure 9). Subsequently, we intersected the TKI-resistant gene set, upregulated differentially expressed genes from bulk RNA data, and genes that were positively correlated with ARHGAP12. We found that the intersection genes were mainly enriched in FA. To validate this finding, we intersected the FA pathway genes obtained with those in the scRNA-seq data, and identified ITGB1 as the unique common gene (Figure 10).
Figure 10.
Potential regulatory mechanisms of Rho GTPase activating protein 12. A: Intersection of tyrosine kinase inhibitor-resistant genes, upregulated differentially expressed genes from bulk high-throughput data, and Rho GTPase activating protein 12 positively correlated genes; B: Enrichment analysis; C: Intersection of genes in the focal adhesion pathway from single-cell RNA sequencing and bulk RNA data. TKI: Tyrosine kinase inhibitor.
The relationship between ARHGAP12 expression and the response to TKIs as well as immunotherapy in HCC
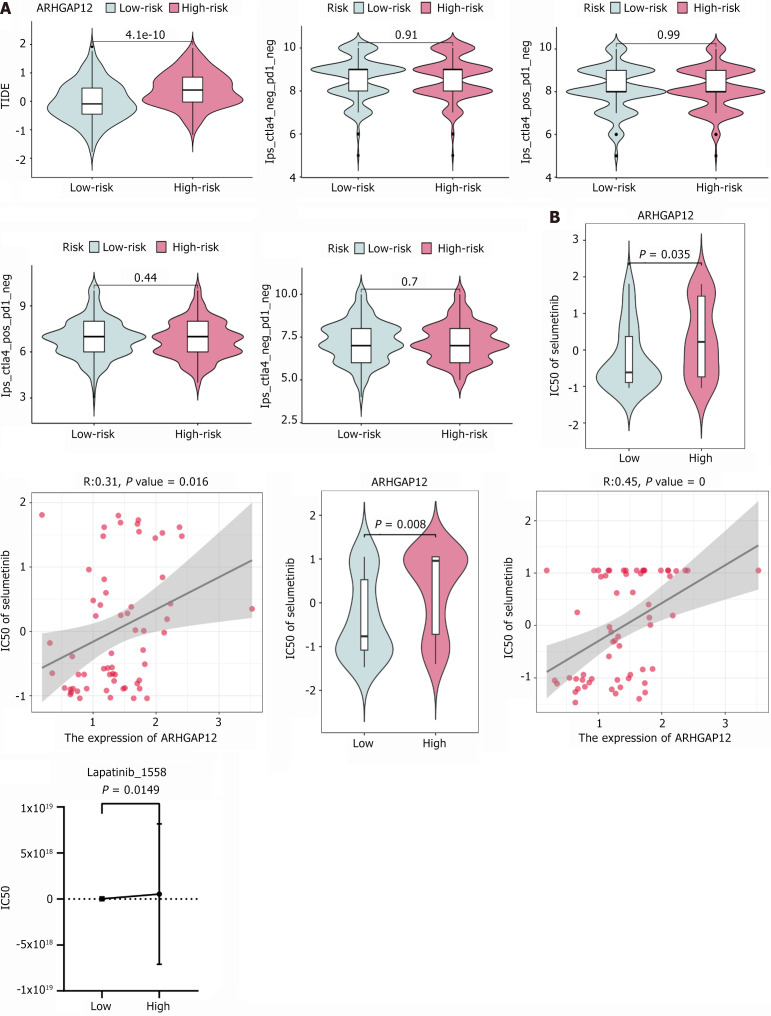
To explore the relationship between ARHGAP12 expression and drug treatment in HCC, we first investigated TCGA data and observed that higher ARHGAP12 expression was associated with increased immune escape. However, no significant correlation was found with immunotherapy response (Figure 11A). Assessment of TKI drug sensitivity revealed that higher ARHGAP12 expression was associated with poorer response to sorafenib, cabozantinib, and regorafenib treatments, with increased dosage requirement correlated with elevated ARHGAP12 expression (P < 0.05, Figure 11B).
Figure 11.
Sensitivity analysis of Rho GTPase activating protein 12 to tyrosine kinase inhibitors and immunotherapy. A: Relationship between Rho GTPase activating protein 12 expression and immune escape, immunotherapy; B: Relationship between Rho GTPase activating protein 12 expression and the intensity of tyrosine kinase inhibitors treatment. ARHGAP12: Rho GTPase activating protein 12.
DISCUSSION
Aberrant expression of ARHGAP12 is a risk factor affecting the prognosis of certain diseases, potentially promoting cell invasion and adhesion[16-18]. In a novel study, we integrated multiple scRNA-seq datasets, confirming that ARHGAP12 mRNA was significantly overexpressed in malignant hepatocytes and uncovering its potential molecular mechanisms through the regulation of the FA pathway. In malignant hepatocytes, the high FA pathway score group predominantly regulated the MK pathway. Notably, within the MK pathway, the MDK-(ITGA6 + ITGB1) ligand- receptor interaction exhibited distinct specificity. Furthermore, by combining bulk RNA, IHC, and proteomics data, we determined that ARHGAP12 mRNA and protein were highly expressed in HCC tissues, with higher expression levels correlating with poorer patient outcomes. This study was the first to report elevated ARHGAP12 expression in TKI-resistant samples, suggesting its potential role as a resistance gene in HCC. The integration of bulk RNA data further revealed that ARHGAP12 might also regulate the FA pathway, with scRNA-seq data identifying ITGB1 as a potential intersecting gene. Our results also showed that higher ARHGAP12 expression was associated with reduced efficacy of lapatinib, selumetinib and trametinib, while it has no significant impact on the efficacy of immunotherapy. Overall, ARHGAP12 appeared to contribute to TKI resistance in HCC by influencing the FA pathway, emerging as a potential key therapeutic target in the treatment of HCC.
Aberrant expression of ARHGAP12 may contribute to disease progression and lead to poor prognosis. In previous studies on ARHGAP12, upregulated miR-199a-5p has been shown to suppress ARHGAP12 expression, leading to decreased urothelial tightness in human ureteral cells and potentially causing bladder pain syndrome[19]. Additionally, in noncoding RNA, overexpression of circular ARHGAP12 promoted the migration and invasion of nasopharyngeal carcinoma cells, accelerating malignant progression through cytoskeletal remodeling[18]. Furthermore, under doxorubicin induction, upregulated circular ARHGAP12 enhanced apoptosis in mouse cardiac tissues[30]. In the genomic analysis of a patient with early-onset colorectal cancer, researchers identified the homozygous missense mutation in the ARHGAP12 gene[20], suggesting that variant expression of ARHGAP12 could be a significant factor promoting colorectal cancer progression from the mutation perspective. Moreover, hepatocyte growth factor played a crucial role in inhibiting the transcription and protein expression of ARHGAP12 during a hepatocyte growth factor-induced invasive growth program[31]. In melanoma, the binding of G-actin to ARHGAP12 could downregulate GAP activity, thereby controlling Rac-dependent signalling pathways and promoting the formation of invasive pseudopodia. Concurrently, high expression of ARHGAP12 has been observed to enhance cell adhesion strength, revealing its dual role in the regulation of cell behavior[22]. While no previous studies have focused on ARHGAP12 in HCC, previous studies on the ARHGAP gene family found that overexpression of ARHGAP18 stimulated growth, migration and invasion of HCC cells[32], while knocking down ARHGAP11A significantly inhibited HCC cell proliferation, and the upregulation of ARHGAP11A promoted mesenchymal-epithelial transition[33]. Interestingly, in the current study, no relationship was found between immunotherapy and ARHGAP12 expression, but high expression of ARHGAP12 resulted in TKI resistance. This suggests the potential significance of ARHGAP12 as a target for TKI treatment. The multi-level and multi-platform exploration conducted in this study strongly suggests that high expression of ARHGAP12 promotes adverse outcomes in HCC and contributes to TKI resistance. Thus, ARHGAP12 may serve as a potential therapeutic target for HCC.
ARHGAP12 may trigger TKI resistance in HCC through FA. Studies have indicated that FAs are large supramolecular complexes closely linked to various cellular behaviors and pathological events, playing a crucial role in interactions between cells and the extracellular matrix as well as the cytoskeleton and regulating cell migration, polarisation and the development of metastatic cancers[34-36]. As a component of FAs, focal adhesion kinase (FAK) plays a central role, functioning as a non-receptor tyrosine kinase and an adaptor protein that plays important roles in processes such as cell adhesion, migration, and survival. Aberrant activation or expression of FAK has been observed in many types of cancers, making it a potential target for cancer therapy and showing particular promise in TKI treatments[37-39]. Other research findings have demonstrated that Ras-related protein Rab-32 and platelet response protein 1 could activate the FAK signalling pathway in non-small cell lung cancer in vitro, conferring resistance to osimertinib and reducing the response to treatment[40]. In osimertinib-resistant lung cancer cell lines, activated FAK was present, and knocking down FAK expression effectively reversed osimertinib resistance mediated by interleukin-6[41]. Interestingly, ARHGAP12 has been shown to have a unique role in regulating cell adhesion. When the expression levels of ARHGAP12 increased, it localized mainly at cell adhesion focal points, significantly advancing the development of the cell adhesion process[21,22]. In the current study, we have extensively explored the role of ARHGAP12 in the mechanism of resistance in HCC for the first time. ARHGAP12 may affect cell adhesion events by modulating the FA pathway, leading to TKI resistance. For example, with increasing ARHGAP12 expression, the efficacy of lapatinib, selumetinib and trametinib diminished. Furthermore, in PPI studies, we found that ARHGAP12 participated in membrane invagination, regulating changes in cell morphology, with alterations in endocytosis accompanying the generation of FAs[42]. This indirectly confirms the potential significant role of FAs in ARHGAP12’s mechanism. This discovery offers a new perspective for addressing TKI resistance in the treatment of HCC.
Targeted therapy of ARHGAP12 may be the key to treating HCC and reversing TKI resistance in HCC. In the complex pathogenesis of HCC, specific molecular targeted therapy provides a new and effective option for patients with advanced HCC[43,44]. As a targeted drug for the treatment of advanced HCC, TKI can inhibit HCC angiogenesis, invasion and metastasis by blocking signalling pathways, such as vascular endothelial growth factor and platelet-derived growth factor[45]. However, clinical data show that up to 70% of HCC patients have poor TKI treatment results, and both primary and acquired TKI resistance have led to limited survival benefits with TKI treatment[46,13]. The discovery of new molecular targets may provide a way to overcome TKI resistance in the treatment of HCC. Recent studies have found that as the first metabolic enzyme in the serine biosynthesis pathway, inactivation of PHGDH could increase reactive oxygen species levels and promote HCC apoptosis after sorafenib treatment. Thus, targeting PHGDH is a potential method to overcome TKI resistance in liver cancer[47]. In addition, upregulated AXL protected resistant cells from oxidative stress, mitochondrial damage and immunogenic cell death by inhibiting tumor necrosis factor-α and STING-type I interferon pathways, which in turn led to poor responses of sorafenib- and lenvatinib-resistant HCC to TKI and immunotherapy. Targeting AXL combined with anti-programmed cell death protein 1 may provide a new option for HCC patients who have failed to respond to TKI treatment[48]. All signs indicate that molecular targeted therapy has great potential in reversing HCC resistance to TKI, and high expression of ARHGAP12 is not only an important driving force for HCC progression but may also be a core factor in the formation of TKI resistance. Therefore, ARHGAP12 is not only of great significance as a potential therapeutic target for HCC, but its targeted inhibition is also likely to become provide a breakthrough in reversing drug resistance and restoring drug sensitivity. Precisely targeting ARHGAP12 is expected to bring new hope for survival to HCC patients whose treatment is limited due to drug resistance. This strategy not only heralds innovation in the treatment model, but is also likely to change the landscape of HCC treatment efficacy.
ARHGAP12 may regulate FA through ITGB1. Integrins, part of the cell adhesion molecule family, are crucial for key interactions between cells and the extracellular matrix, playing essential roles in cell adhesion, spreading, and migration. Integrins act as bridges between the extracellular matrix and the Rho GTPase family, transmitting extracellular information to the cell nucleus via the actin cytoskeleton. For example, ITGB1 regulated actin dynamics through Ras homolog A[17,49,50]. Integrins containing ITGB1 as a subunit played indispensable roles in various cell signalling pathways to maintain normal physiological functions. However, in the context of tumor cells, the roles of integrins underwent transformation as they not only contributed to the maintenance of stemness in tumor cells but also enhanced invasion, metastasis, and chemotherapy resistance. Notably, ITGB1, as a critical subunit of integrin proteins, could bind to multiple ligands, exhibiting diverse biological functions[51]. As the core of the integrin family, ITGB1’s adhesive function has been confirmed to be the key mechanism for disease progression[52]. Lysyl oxidase-like 2 has been shown to stabilize the protein structure of ITGB1 and activate the FAK/SRC signalling pathway mediated by ITGB1, significantly accelerating the formation of FAs. This process enhanced the pro-migratory function of ribosomal protein S7, promoting cell migration and invasion[53]. Elevated expression of ITGB1, a key gene in collagen-related cell adhesion molecules, could promote HCC progression[54]. Current research suggests that ITGB1 has a role in regulating FAs in HCC. The current study discovered a potential regulatory gene, ARHGAP12, in the FA pathway. Regarding FAs, ARHGAP12 may potentially upregulate ITGB1 to increase cell adhesion levels, promote HCC progression and, consequently, trigger TKI resistance, leading to poor prognosis. However, further investigation is required to determine the potential synergistic regulation of FAs by ARHGAP12 and ITGB1.
Our study has several limitations. First, clinical samples matter greatly to the research, and it is necessary to increase the sample size, which would be beneficial for this study. Secondly, additional adhesion-related research on this gene will enhance our understanding of ARHGAP12’s clinical value, facilitating a better evaluation of its clinical significance. Moreover, the study is dependent on retrospective data and potential selection bias might exist. Increasing relevant clinical and animal experiments can offer better proof of our viewpoints.
CONCLUSION
Our study reveals the high expression of ARHGAP12 in HCC, which may trigger TKI resistance, possibly through its regulatory role in FA. Overall, our research unveils molecular changes in HCC, linking ARHGAP12 with HCC and TKI resistance, providing new insights for the treatment of TKI-resistant HCC in the future.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Guangxi Zhuang Autonomous Region Clinical Medicine Research Center for Molecular Pathology and Intelligent Pathology Precision Diagnosis for skillful technical assistance.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University Institutional Review Board, Approval No. 2022-KT-Guoji-127.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Zhang J S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
Contributor Information
Xiao-Wei Wang, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Yu-Xing Tang, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Fu-Xi Li, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Jia-Le Wang, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Gao-Peng Yao, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Da-Tong Zeng, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China; Department of Pathology, Red Cross Hospital of Yulin City, Yulin 537000, Guangxi Zhuang Autonomous Region, China.
Yu-Lu Tang, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Bang-Teng Chi, Department of Medical Oncology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Qin-Yan Su, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Lin-Qing Huang, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Di-Yuan Qin, Department of Computer Science and Technology, School of Computer and Electronic Information, Guangxi University, Nanning 530004, Guangxi Zhuang Autonomous Region, China.
Gang Chen, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Zhen-Bo Feng, Department of Pathology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China. fengzhenbo_gxmu@163.com.
Rong-Quan He, Department of Medical Oncology, The First Affiliated Hospital of Guangxi Medical University, Nanning 530021, Guangxi Zhuang Autonomous Region, China.
Data sharing statement
Dataset available from the co-first author at tang_yx_patho@163.com. Participants gave informed consent for data sharing.
References
- 1.Zheng RS, Chen R, Han BF, Wang SM, Li L, Sun KX, Zeng HM, Wei WW, He J. [Cancer incidence and mortality in China, 2022] Zhonghua Zhong Liu Za Zhi. 2024;46:221–231. doi: 10.3760/cma.j.cn112152-20240119-00035. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Huang M, Zhao Z, Yang L. Long noncoding RNA small nucleolar RNA host genes as prognostic molecular biomarkers in hepatocellular carcinoma: A meta-analysis. Cancer Med. 2024;13:e7200. doi: 10.1002/cam4.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu PY, Tung SL, Tsai KW, Shen FP, Chan SH. Identification of the Novel Oncogenic Role of SAAL1 and Its Therapeutic Potential in Hepatocellular Carcinoma. Cancers (Basel) 2020;12 doi: 10.3390/cancers12071843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta T, Jarpula NS. Hepatocellular carcinoma immune microenvironment and check point inhibitors-current status. World J Hepatol. 2024;16:353–365. doi: 10.4254/wjh.v16.i3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Zhang W, Bi X, Yang Z, Tang Y, Jiang L, Bi F, Chen M, Cheng S, Chi Y, Han Y, Huang J, Huang Z, Ji Y, Jia L, Jiang Z, Jin J, Jin Z, Li X, Li Z, Liang J, Liu L, Liu Y, Lu Y, Lu S, Meng Q, Niu Z, Pan H, Qin S, Qu W, Shao G, Shen F, Song T, Song Y, Tao K, Tian A, Wang J, Wang W, Wang Z, Wu L, Xia F, Xing B, Xu J, Xue H, Yan D, Yang L, Ying J, Yun J, Zeng Z, Zhang X, Zhang Y, Zhang Y, Zhao J, Zhou J, Zhu X, Zou Y, Dong J, Fan J, Lau WY, Sun Y, Yu J, Zhao H, Zhou A, Cai J. Systemic Therapy for Hepatocellular Carcinoma: Chinese Consensus-Based Interdisciplinary Expert Statements. Liver Cancer. 2022;11:192–208. doi: 10.1159/000521596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Praveen Kumar PK, Sundar H, Balakrishnan K, Subramaniam S, Ramachandran H, Kevin M, Michael Gromiha M. The Role of HSP90 and TRAP1 Targets on Treatment in Hepatocellular Carcinoma. Mol Biotechnol. 2024 doi: 10.1007/s12033-024-01151-4. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Peng G, Li J. A Network Meta-analysis of the Efficacy of Drug Therapy in First-line Treatment of Advanced Hepatocellular Carcinoma. J Gastrointestin Liver Dis. 2024;33:94–101. doi: 10.15403/jgld-5289. [DOI] [PubMed] [Google Scholar]
- 10.Lei J, Chen B, Song M, Zhang L, Zhang X, Gao X, Li Y, Lu Y, Zuo S. TKI or TKI combined with PD-1 inhibitors as second-line treatment for HCC patients after sorafenib failure. Front Pharmacol. 2022;13:1026337. doi: 10.3389/fphar.2022.1026337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tümen D, Heumann P, Gülow K, Demirci CN, Cosma LS, Müller M, Kandulski A. Pathogenesis and Current Treatment Strategies of Hepatocellular Carcinoma. Biomedicines. 2022;10 doi: 10.3390/biomedicines10123202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M, Liu Y, Wan HL, Wong AM, Ding X, You W, Lo WS, Ng KK, Wong N. Overexpression of nucleotide metabolic enzyme DUT in hepatocellular carcinoma potentiates a therapeutic opportunity through targeting its dUTPase activity. Cancer Lett. 2022;548:215898. doi: 10.1016/j.canlet.2022.215898. [DOI] [PubMed] [Google Scholar]
- 13.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 14.Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, Ying J, Lu Y, Meng Z, Pan H, Yang P, Zhang H, Chen X, Xu A, Cui C, Zhu B, Wu J, Xin X, Wang J, Shan J, Chen J, Zheng Z, Xu L, Wen X, You Z, Ren Z, Liu X, Qiu M, Wu L, Chen F. Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial. J Clin Oncol. 2021;39:3002–3011. doi: 10.1200/JCO.21.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan D, Liu H, Qu P, Chen X, Ma X, Wang Y, Qin X, Han Z. Comparing Safety and Efficacy of TACE + Apatinib in Combination with a PD-1 Inhibitor versus a Non-triple Therapy for Treating Advanced Primary Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. J Gastrointestin Liver Dis. 2024;33:85–93. doi: 10.15403/jgld-5159. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Wu C, Wang S, Huang W, Zhou Z, Ying K, Xie Y, Mao Y. Cloning and characterization of ARHGAP12, a novel human rhoGAP gene. Int J Biochem Cell Biol. 2002;34:325–331. doi: 10.1016/s1357-2725(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 17.Guenther C. β2-Integrins - Regulatory and Executive Bridges in the Signaling Network Controlling Leukocyte Trafficking and Migration. Front Immunol. 2022;13:809590. doi: 10.3389/fimmu.2022.809590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan C, Qu H, Xiong F, Tang Y, Tang T, Zhang L, Mo Y, Li X, Guo C, Zhang S, Gong Z, Li Z, Xiang B, Deng H, Zhou M, Liao Q, Zhou Y, Li X, Li Y, Li G, Wang F, Zeng Z. CircARHGAP12 promotes nasopharyngeal carcinoma migration and invasion via ezrin-mediated cytoskeletal remodeling. Cancer Lett. 2021;496:41–56. doi: 10.1016/j.canlet.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Monastyrskaya K, Sánchez-Freire V, Hashemi Gheinani A, Klumpp DJ, Babiychuk EB, Draeger A, Burkhard FC. miR-199a-5p regulates urothelial permeability and may play a role in bladder pain syndrome. Am J Pathol. 2013;182:431–448. doi: 10.1016/j.ajpath.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Tanskanen T, Gylfe AE, Katainen R, Taipale M, Renkonen-Sinisalo L, Järvinen H, Mecklin JP, Böhm J, Kilpivaara O, Pitkänen E, Palin K, Vahteristo P, Tuupanen S, Aaltonen LA. Systematic search for rare variants in Finnish early-onset colorectal cancer patients. Cancer Genet. 2015;208:35–40. doi: 10.1016/j.cancergen.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Bae DJ, Seo J, Kim SY, Park SY, Do Yoo J, Pyo JH, Cho W, Cho JY, Kim S, Kim IS. ArhGAP12 plays dual roles in Stabilin-2 mediated efferocytosis: Regulates Rac1 basal activity and spatiotemporally turns off the Rac1 to orchestrate phagosome maturation. Biochim Biophys Acta Mol Cell Res. 2019;1866:1595–1607. doi: 10.1016/j.bbamcr.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Diring J, Mouilleron S, McDonald NQ, Treisman R. RPEL-family rhoGAPs link Rac/Cdc42 GTP loading to G-actin availability. Nat Cell Biol. 2019;21:845–855. doi: 10.1038/s41556-019-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaehler M, Litterst M, Kolarova J, Böhm R, Bruckmueller H, Ammerpohl O, Cascorbi I, Nagel I. Genomewide expression and methylation analyses reveal aberrant cell adhesion signaling in tyrosine kinase inhibitorresistant CML cells. Oncol Rep. 2022;48 doi: 10.3892/or.2022.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Y, Yang A, Quan C, Pan Y, Zhang H, Li Y, Gao C, Lu H, Wang X, Cao P, Chen H, Lu S, Zhou G. A single-cell atlas of the multicellular ecosystem of primary and metastatic hepatocellular carcinoma. Nat Commun. 2022;13:4594. doi: 10.1038/s41467-022-32283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li JD, Chen Y, Jing SW, Wang LT, Zhou YH, Liu ZS, Song C, Li DZ, Wang HQ, Huang ZG, Dang YW, Chen G, Luo JY. Triosephosphate isomerase 1 may be a risk predictor in laryngeal squamous cell carcinoma: a multi-centered study integrating bulk RNA, single-cell RNA, and protein immunohistochemistry. Eur J Med Res. 2023;28:591. doi: 10.1186/s40001-023-01568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong K, Fang Y, Qiu B, Chen C, Huang N, Liang F, Huang C, Lu T, Zheng L, Zhao J, Zhu B. Investigation of cellular communication and signaling pathways in tumor microenvironment for high TP53-expressing osteosarcoma cells through single-cell RNA sequencing. Med Oncol. 2024;41:93. doi: 10.1007/s12032-024-02318-4. [DOI] [PubMed] [Google Scholar]
- 27.Li GS, Tang YX, Zhang W, Li JD, Huang HQ, Liu J, Fu ZW, He RQ, Kong JL, Zhou HF, Chen G. MMP12 is a Potential Predictive and Prognostic Biomarker of Various Cancers Including Lung Adenocarcinoma. Cancer Control. 2024;31:10732748241235468. doi: 10.1177/10732748241235468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y, Su F, Yang H, Xiao Y, Zhang X, Su H, Zhang T, Bai Y, Ling X. E2F1 transcriptionally regulates CCNA2 expression to promote triple negative breast cancer tumorigenicity. Cancer Biomark. 2022;33:57–70. doi: 10.3233/CBM-210149. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Liang ZQ, He RQ, Huang ZG, Wang XM, Wei MY, Su HL, Liu ZS, Zheng YS, Huang WY, Zhang HJ, Dang YW, Li SH, Cheng JW, Chen G, He J. The upregulation and transcriptional regulatory mechanisms of Extra spindle pole bodies like 1 in bladder cancer: An immunohistochemistry and high-throughput screening Evaluation. Heliyon. 2024;10:e31192. doi: 10.1016/j.heliyon.2024.e31192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Cheng Z, Xu J, Feng M, Zhang H, Zhang L, Qian L. Circular RNA Arhgap12 modulates doxorubicin-induced cardiotoxicity by sponging miR-135a-5p. Life Sci. 2021;265:118788. doi: 10.1016/j.lfs.2020.118788. [DOI] [PubMed] [Google Scholar]
- 31.Gentile A, D'Alessandro L, Lazzari L, Martinoglio B, Bertotti A, Mira A, Lanzetti L, Comoglio PM, Medico E. Met-driven invasive growth involves transcriptional regulation of Arhgap12. Oncogene. 2008;27:5590–5598. doi: 10.1038/onc.2008.173. [DOI] [PubMed] [Google Scholar]
- 32.Chen P, Liu X, Liu Y, Bao X, Wu Q. ARHGAP18 is Upregulated by Transcription Factor GATA1 Promotes the Proliferation and Invasion in Hepatocellular Carcinoma. Appl Biochem Biotechnol. 2024;196:679–689. doi: 10.1007/s12010-023-04459-0. [DOI] [PubMed] [Google Scholar]
- 33.Dai B, Zhang X, Shang R, Wang J, Yang X, Zhang H, Liu Q, Wang D, Wang L, Dou K. Blockade of ARHGAP11A reverses malignant progress via inactivating Rac1B in hepatocellular carcinoma. Cell Commun Signal. 2018;16:99. doi: 10.1186/s12964-018-0312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumacher S, Vazquez Nunez R, Biertümpfel C, Mizuno N. Bottom-up reconstitution of focal adhesion complexes. FEBS J. 2022;289:3360–3373. doi: 10.1111/febs.16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang X, Wu X, Guo T, Jia F, Hu Y, Xing X, Gao X, Li Z. Focal Adhesion-Related Signatures Predict the Treatment Efficacy of Chemotherapy and Prognosis in Patients with Gastric Cancer. Front Oncol. 2022;12:808817. doi: 10.3389/fonc.2022.808817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao G, Luo T, Liu Z, Li J. Development and validation of focal adhesion-related genes signature in gastric cancer. Front Genet. 2023;14:1122580. doi: 10.3389/fgene.2023.1122580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen S, He T, Zhong Y, Chen M, Yao Q, Chen D, Shao Z, Xiao G. Roles of focal adhesion proteins in skeleton and diseases. Acta Pharm Sin B. 2023;13:998–1013. doi: 10.1016/j.apsb.2022.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Li J, Jiao S, Han G, Zhu J, Liu T. Functional and clinical characteristics of focal adhesion kinases in cancer progression. Front Cell Dev Biol. 2022;10:1040311. doi: 10.3389/fcell.2022.1040311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan X, Yan Y, Song B, Zhu S, Mei Q, Wu K. Focal adhesion kinase: from biological functions to therapeutic strategies. Exp Hematol Oncol. 2023;12:83. doi: 10.1186/s40164-023-00446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosibaty Z, Brustugun OT, Zwicky Eide IJ, Tsakonas G, Grundberg O, De Petris L, McGowan M, Hydbring P, Ekman S. Ras-Related Protein Rab-32 and Thrombospondin 1 Confer Resistance to the EGFR Tyrosine Kinase Inhibitor Osimertinib by Activating Focal Adhesion Kinase in Non-Small Cell Lung Cancer. Cancers (Basel) 2022;14 doi: 10.3390/cancers14143430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L, Li Z, Lu C, Li J, Zhang K, Lin C, Tang X, Liu Z, Zhang Y, Han R, Wang Y, Feng M, Zhuang Y, Hu C, He Y. Ibrutinib reverses IL-6-induced osimertinib resistance through inhibition of Laminin α5/FAK signaling. Commun Biol. 2022;5:155. doi: 10.1038/s42003-022-03111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.di Blasio L, Gagliardi PA, Puliafito A, Sessa R, Seano G, Bussolino F, Primo L. PDK1 regulates focal adhesion disassembly by modulating endocytosis of αvβ3 integrin. J Cell Sci. 2015;128:863–877. doi: 10.1242/jcs.149294. [DOI] [PubMed] [Google Scholar]
- 43.Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, Wang C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:203–222. doi: 10.1038/s41575-022-00704-9. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Deng B. Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023;42:629–652. doi: 10.1007/s10555-023-10084-4. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y, Zhang YN, Wang KT, Chen L. Lenvatinib for hepatocellular carcinoma: From preclinical mechanisms to anti-cancer therapy. Biochim Biophys Acta Rev Cancer. 2020;1874:188391. doi: 10.1016/j.bbcan.2020.188391. [DOI] [PubMed] [Google Scholar]
- 46.Jiang N, Xie B, Xiao W, Fan M, Xu S, Duan Y, Hamsafar Y, Evans AC, Huang J, Zhou W, Lin X, Ye N, Wanggou S, Chen W, Jing D, Fragoso RC, Dugger BN, Wilson PF, Coleman MA, Xia S, Li X, Sun LQ, Monjazeb AM, Wang A, Murphy WJ, Kung HJ, Lam KS, Chen HW, Li JJ. Fatty acid oxidation fuels glioblastoma radioresistance with CD47-mediated immune evasion. Nat Commun. 2022;13:1511. doi: 10.1038/s41467-022-29137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei L, Lee D, Law CT, Zhang MS, Shen J, Chin DW, Zhang A, Tsang FH, Wong CL, Ng IO, Wong CC, Wong CM. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC. Nat Commun. 2019;10:4681. doi: 10.1038/s41467-019-12606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xie Y, Wu H, He Y, Liu L, Huang IB, Zhou L, Lin CY, Leung RW, Loh JJ, Lee TK, Ding J, Man K, Ma S, Tong M. Targeting AXL induces tumor-intrinsic immunogenic response in tyrosine kinase inhibitor-resistant liver cancer. Cell Death Dis. 2024;15:110. doi: 10.1038/s41419-024-06493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu W, Sun H, Zhang M, Mo S, Tan C, Ni S, Yang Z, Wang Y, Sheng W, Wang L. ITGB1 as a prognostic biomarker correlated with immune suppression in gastric cancer. Cancer Med. 2023;12:1520–1531. doi: 10.1002/cam4.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S, Sampson C, Liu C, Piao HL, Liu HX. Integrin signaling in cancer: bidirectional mechanisms and therapeutic opportunities. Cell Commun Signal. 2023;21:266. doi: 10.1186/s12964-023-01264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su C, Mo J, Dong S, Liao Z, Zhang B, Zhu P. Integrinβ-1 in disorders and cancers: molecular mechanisms and therapeutic targets. Cell Commun Signal. 2024;22:71. doi: 10.1186/s12964-023-01338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun L, Guo S, Xie Y, Yao Y. The characteristics and the multiple functions of integrin β1 in human cancers. J Transl Med. 2023;21:787. doi: 10.1186/s12967-023-04696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou YJ, Yang ML, He X, Gu HY, Ren JH, Cheng ST, Fu Z, Zhang ZZ, Chen J. RNA-binding protein RPS7 promotes hepatocellular carcinoma progression via LOXL2-dependent activation of ITGB1/FAK/SRC signaling. J Exp Clin Cancer Res. 2024;43:45. doi: 10.1186/s13046-023-02929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Hu Y, Zhao K, Fan J, Zhu J, Li Q, Chen Q, Lu Y. Comprehensive Analysis of the Expression of Cell Adhesion Molecules Genes in Hepatocellular Carcinoma and their Prognosis, and Biological Significance. Front Biosci (Landmark Ed) 2024;29:76. doi: 10.31083/j.fbl2902076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Dataset available from the co-first author at tang_yx_patho@163.com. Participants gave informed consent for data sharing.