Abstract
Background
Interstitial lung disease (ILD) is a serious complication in systemic juvenile idiopathic arthritis (SJIA). This study aimed to identify the clinical characteristics and prognosis of SJIA-ILD.
Methods
A two-center retrospective cohort study was conducted on patients newly diagnosed with SJIA in China from October 2010 to December 2021. Clinical characteristics, laboratory parameters, outcomes, and relapse rates were compared between ILD and non-ILD groups.
Results
A total of 176 children with SJIA were included, including 35 in ILD group and 141 in non-ILD group. The median age at onset of SJIA was 5.8 years (range 4.4–9.5) in patients with SJIA-ILD. It exhibited higher incidences of cervical spine (28.6%) and hip involvement (40.0%) in ILD group (P = 0.031 and P = 0.029, respectively). The incidence of macrophage activation syndrome (MAS) in ILD group reached up to 40%, significantly elevated than that in non-ILD group (P = 0.047). Children with ILD demonstrated a stronger inflammatory response and were more prone to developing lymphopenia (P = 0.009), requiring more combination therapy (P = 0.006) to control disease activity. 54.3% of patients received biologic therapies, with only three patient receiving biologics (one with IL-6 blockade, two with TNF inhibitor) prior to ILD onset and none receiving IL-1 blockade. The median follow-up duration was 6.0 years (range 3.9–9.5). The proportions of patients with SJIA-ILD achieving clinical inactive disease without glucocorticoids within 6 to 12 months of the treatment were significantly lower than control group (45.7% vs. 70.2%, P = 0.006). In ILD group, only 54.3% of patients achieved complete remission, and 17.1% were in a non-remission state, among whom two deaths from respiratory failure. There was no significant difference in disease relapse rates between the two groups (P > 0.05).
Conclusions
Patients with SJIA-ILD exhibited heightened inflammation, increased hip joint and cervical spine involvement, and were more susceptible to developing lymphopenia and MAS, suggesting a relatively poor prognosis. They required a prolonged time to control inflammation and more aggressive treatment strategies to achieve inactive status. The unsatisfactory rate of complete remission highlighted an urgent need for focused clinical strategies.
Keywords: Juvenile idiopathic arthritis, Interstitial lung disease, Pediatrics, Rheumatology
Introduction
Systemic juvenile idiopathic arthritis (SJIA) is a rare and severe subtype of JIA characterized by systemic features such as persistent high fever, evanescent rash, arthritis, and organomegaly [1]. Unlike other subtypes of JIA, SJIA is associated with a complex pathogenesis that involves innate immune disorder and the presence of multiple pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6 and IL-18 [2], which can cause more severe systemic complications. For example, macrophage activation syndrome (MAS), a life-threatening hematological condition, primarily occurs in children with SJIA [3].
Recently, more and more studies have focused on interstitial lung disease (ILD), another potential fatal complication in patients with SJIA [4, 5]. Huang et al. [6] reported a series of 41 patients with SJIA and lung diseases including ILD, lipoid pneumonia, and pulmonary arterial hypertension, which could result in a high mortality rate. A recent cohort survey summarized high-risk factors for ILD, including a history of MAS, young age at diagnosis, high IL-18 levels, trisomy 21, and adverse reactions to biological disease-modifying anti-rheumatic drugs (bDMARDs) medication [4, 5, 7]. However, ILD may sometimes have an insidious onset, characterized by nonspecific respiratory symptoms, including dyspnea, shortness of breath, and coughing [7], which can easily lead to misdiagnosis.
Early diagnosis and appropriate treatment are crucial for improving the prognosis of the disease and preventing the occurrence of complication. Recent studies have demonstrated a strong association between the use of IL-1 and IL-6 inhibitors and the development of ILD in patients with SJIA [5, 8]. Given different treatment landscape, this cohort study focused on the clinical presentations and outcomes of patients with SJIA-ILD to help raise awareness and recognition of this disease in the Chinese pediatric population.
Materials and methods
Study population
Data of newly diagnosed patients with SJIA from two children’s hospitals in China between October 2010 and December 2021 were included in this study. Patients diagnosed with SJIA met the criteria of the International League of Rheumatology Alliance (ILAR) [9] or 2018 PRINTO classification criteria [10] and all patients were followed up for more than 2 years. MAS was diagnosed based on 2016 Ravelli classification criteria [11]. ILD was diagnosed based on chest high-resolution computed tomography (HRCT) according the criteria for childhood interstitial lung disease (ChILD) [12]. The exclusion criteria were as follows: (1) malignant tumor, (2) infection-related ILD, (3) bronchopulmonary dysplasia, immotile cilia syndrome, and congenital ILD, (4) metabolic diseases: such as glycogen accumulation disease, and (5) primary immunodeficiency disease.
The study protocol was approved by the ethics committee of the Second Affiliated Hospital of Wenzhou Medical University (No. 2021-K-258-02) and the Children’s Hospital of Nanjing Medical University (No. 20150629-1), which waived the requirement for informed consent from all patients’ parents.
Data collection
Patients were identified from electronic medical records from October 2010 to December 2021 with the diagnostic codes for SJIA. All diagnoses were confirmed by two experienced radiologist and two pediatric rheumatologists. Data, including sex, age of onset, clinical presentation, laboratory tests, comorbidities, and treatments, was collected from the electronic medical record systems of both hospitals. Disease activity in SJIA was assessed using the Juvenile Arthritis Disease Activity Score-27 (JADAS-27) score [13], which comprises four measures: active joint count (covering 27 joints), physician global assessment of disease activity (measured on a 10-cm visual analog scale (VAS)), parent/patient global assessment of well-being (measured on a 10-cm VAS), and erythrocyte sedimentation rate (ESR). According to the Wallace criteria [14], clinical inactive disease (CID) criteria included the satisfaction of all of the following points: (1) no active arthritis; (2) no fever, rash, serositis, splenomegaly, or generalized lymphadenopathy; (3) no active uveitis; (4) ESR ≤ 20 mm/h and/or C-reactive protein (CRP) ≤ 10 mg/L; and (5) physician’s global assessment of disease activity reflecting inactive status.
Treatment targets included the short-term (Goal one), the mid-term (Goal two), and the long-time target (Goal three). Goal one aimed to resolve of fever, and decrease in C-reactive protein by 50% within 7 days. Goal two aimed to improve physician global and active joint count by at least 50% or a JADAS-10 score of maximally 5.4 within 4 weeks. Goal three aimed to achieve CID without glucocorticoids within 6 to 12 months of the treatment.
Prognostic assessment criterias were as follows [15]: (1) complete remission (CR) was defined as sustained CID for a duration exceeding 6 months under medication treatment or for more than 12 months after discontinuation of medication, (2) partial remission (PR) was defined as meeting American College of Rheumatology (ACR) 30, 50 or 70 standards and not achieving the inactive state, (3) no remission (NR) was defined as not reaching ACR30 response despite treatment, and (4) death. Relapse was defined as a 30% or more deterioration in at least three ACR indicators, accompanied by an absence of improvement in any indicator. The evaluation of most relapsed patients was conducted during hospitalization. Only those with detailed and complete records were included in the study cohort.
Statistical analysis
Data were analyzed using the SPSS package (version 27.0; SPSS Inc., Chicago, IL, USA). Continuous variables were presented as mean ± standard deviation (SD) or median (interquartile range). Categorical variables were presented as numbers and proportions. When comparing the differences in quantitative variables between SJIA with ILD group and non-ILD group, we used the Student’s t-test for univariate analysis according to the data distribution. For the bivariate analysis of qualitative variables, the Pearson’s chi-square test, Yates’ correction for continuity, or Fisher’s exact test of continuity was used. Statistical significance was defined as a two-sided P-value < 0.05.
Results
Baseline characteristic and clinical presentation
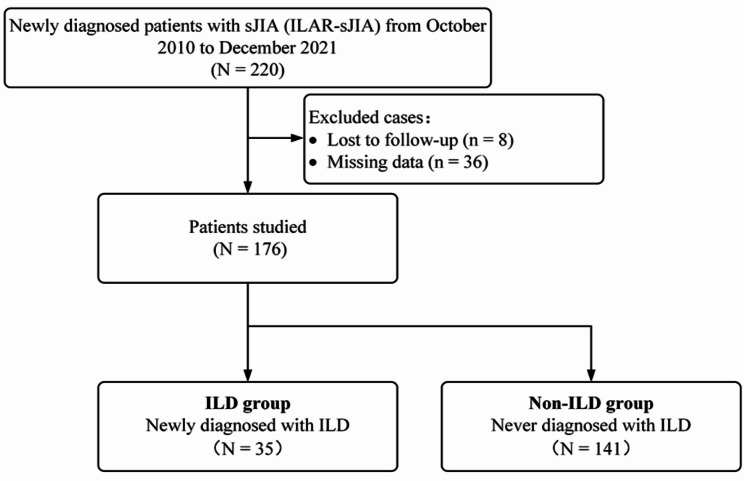
176 patients with SJIA who met the inclusion criteria were included in this study from two centers after excluding cases with incomplete data or those lost to follow-up. 35 patients were included in ILD group and 141 patients in non-ILD group, respectively (Fig. 1).
Fig. 1.
Flowchart of the study design
Baseline characteristics, clinical presentations, median JADAS-27 score at onset of SJIA and MAS episode were presented in Table 1. The median age at onset of SJIA was 5.8 years (range 4.4–9.5) in the ILD group and 6.8 years (range 4.7–9.6) in the non-ILD group. In the ILD group, 51.4% were female and 48.6% were male. There were no significant differences of age or sex between the two groups (P = 0.864 and P = 0.679, respectively).
Table 1.
Baseline characteristic and clinical presentation of patients with SJIA
| ILD (n = 35) |
Non-ILD (n = 141) |
P value | |
|---|---|---|---|
| Median age at onset(y) | 5.8 (4.4–9.5) | 6.8 (4.7–9.6) | 0.864 |
| Sex, n (%) | |||
| Male | 17 (48.6) | 74 (52.5) | 0.679 |
| Female | 18 (51.4) | 67 (47.5) | |
| Median follow-up duration(y) | 4.6 (3.7–10.2) | 6.3 (4.1–9.5) | 0.859 |
| Hepatosplenomegaly, n (%) | 19 (54.3) | 52 (36.9) | 0.060 |
| Arthritis, n (%) | 30 (85.7) | 112 (79.4) | 0.399 |
| TMJ | 1 (2.9) | 3 (2.1) | 1.000 |
| Cervical spine | 10 (28.6) | 19 (13.5) | 0.031* |
| Shoulder | 11 (31.4) | 28 (19.9) | 0.140 |
| Elbow | 10 (28.6) | 32 (22.7) | 0.465 |
| Wrists | 16 (45.7) | 47 (33.3) | 0.171 |
| Hip | 14 (40.0) | 31 (22.0) | 0.029* |
| Knee | 22 (62.9) | 84 (59.6) | 0.722 |
| Ankle | 12 (34.3) | 62 (44.0) | 0.299 |
| Knuckle and toe joints | 8 (22.9) | 35 (24.8) | 0.809 |
| MAS, n (%) | 14 (40.0) | 33 (23.4) | 0.047* |
| One episode | 10 (28.6) | 29 (20.6) | 0.307 |
| ≥ 2 episodes | 4 (11.4) | 4 (2.8) | 0.083 |
| Median JADAS-27 score | 15.0 (11.0–18.13) | 13.2 (9.3–16.5) | 0.244 |
TMJ temporomandibular joint, MAS macrophage activation syndrome. *P < 0.05
142 patients (80.7%) diagnosed with SJIA presented with arthritis. Hepatosplenomegaly was present in 54.3% of patients in the ILD group and 36.9% of patients in the non-ILD group. There were no significant differences in hepatosplenomegaly or arthritis between the two groups (P = 0.060 and P = 0.399, respectively). However, the ILD group exhibited a markedly greater incidence of cervical spine and hip involvement compared to the non-ILD group (P = 0.031 and P = 0.029, respectively). The frequency of MAS in ILD group was significantly elevated than that in non-ILD group (40.0% vs. 23.4%, P = 0.047). The median JADAS-27 score was 15.0 vs. 13.2 in the two groups. Among 35 patients with SJIA-ILD, 21 patients presented with ILD at the onset of SJIA, while 14 developed ILD later in disease course. 31 cases (89%) had respiratory symptoms at ILD diagnosis, including 26 cases with dry cough, 25 cases with tachypnea or dyspnea, 4 cases with chest pain, and 4 cases with digital clubbing, while 4 patients presented insidious without typical respiratory symptoms. 10 patients who progressed to respiratory support,10 high-flow nasal cannula, 2 non-invasive ventilation and 2 mechanical ventilation.
Laboratory test
Inflammatory markers were significantly elevated at the onset of the disease. However, pediatric patients with ILD demonstrated a stronger inflammatory response, including peripheral white blood cell (WBC) count, absolute neutrophil count (ANC), and CRP level, all of which were significantly higher than those in the non-ILD group (P = 0.006, P = 0.003 and P = 0.000, respectively). Significantly increased serum ferritin (SF) level, another crucial inflammatory marker during SJIA activity, was also observed in the ILD group compared to the non-ILD group (P = 0.018). The proportion of ferritin absolute values greater than 684 ng/mL (the cutoff value in MAS diagnostic criteria) was similarly significantly higher in the ILD group (P = 0.006). Of concern, ILD patients were more prone to develop lymphopenia (absolute lymphocyte count < 1 × 109/L) at the onset of the disease (P = 0.009). Elevated levels of inflammatory cytokines IL-6, IL-10, and tumor necrosis factor-α (TNF-α) were detected in the ILD group compared to the non-ILD group, but there is no significant difference between the two groups (Table 2).
Table 2.
Laboratory characteristics during the onset of the SJIA
| ILD (n = 35) |
Non-ILD (n = 141) |
P value | |
|---|---|---|---|
| ESR (mm/h) | 74.5 ± 27.3 | 68.9 ± 26.3 | 0.267 |
| SF (ng/mL) | 2622.7 ± 3407.9 | 1147.4 ± 1668.1 | 0.018* |
| SF > 684 ng/mL, n (%) | 27 (77.1) | 68 (51.5) | 0.006** |
| PCT (ng/mL) | 3.4 ± 9.2 | 0.7 ± 2.7 | 0.137 |
| CRP (mg/L) | 122.2 ± 62.8 | 79.4 ± 50.8 | 0.000*** |
| WBC (×109/L) | 24.0 ± 12.8 | 17.3 ± 8.6 | 0.006** |
| ANC (×109/L) | 20.4 ± 11.8 | 13.6 ± 8.2 | 0.003** |
| ALC (×109/L) | 2.2 ± 1.1 | 2.8 ± 1.5 | 0.039* |
| Lymphopenia#, n (%) | 18 (51.4) | 40 (28.4) | 0.009** |
| ALT (U/L) | 34.1 ± 36.9 | 43.7 ± 90.4 | 0.537 |
| AST (U/L) | 32.3 ± 15.3 | 45.6 ± 82.3 | 0.343 |
| ALP (U/L) | 156.9 ± 63.9 | 170.7 ± 55.4 | 0.204 |
| GGT (U/L) | 68.5 ± 82.3 | 39.9 ± 49.6 | 0.056 |
| Total protein (g/L) | 66.4 ± 7.1 | 69.0 ± 6.9 | 0.045* |
| LDH (U/L) | 586.9 ± 571.8 | 399.0 ± 486.5 | 0.053 |
| Serum calcium (mmol/L) | 2.2 ± 0.1 | 2.3 ± 0.2 | 0.082 |
| Fibrinogen (g/L) | 5.97 ± 2.12 | 6.59 ± 3.37 | 0.312 |
| TG (mmol/L) | 1.3 ± 0.6 | 1.2 ± 0.7 | 0.156 |
| IL-6 (pg/mL) | 147.8 ± 372.7 | 41.8 ± 73.1 | 0.221 |
| IL-10 (pg/mL) | 12.6 ± 14.2 | 8.9 ± 10.4 | 0.201 |
| TNF-α (pg/mL) | 66.5 ± 214.8 | 15.1 ± 17.6 | 0.298 |
| CD3+ (%) | 69.4 ± 12.6 | 70.7 ± 10.4 | 0.566 |
| CD4 (%) | 36.4 ± 10.1 | 36.6 ± 10.0 | 0.915 |
| CD8 (%) | 29.2 ± 8.3 | 29.8 ± 10.9 | 0.778 |
| CD16 + 56+ (%) | 10.1 ± 7.8 | 10.4 ± 7.0 | 0.828 |
| CD19 (%) | 17.6 ± 10.0 | 16.7 ± 8.3 | 0.618 |
| IgG (mg/dL) | 526.5 ± 645.1 | 563.3 ± 666.6 | 0.775 |
| IgA (mg/dL) | 98.6 ± 137.7 | 93.4 ± 121.7 | 0.835 |
| IgM (mg/dL) | 67.8 ± 81.1 | 73.3 ± 89.5 | 0.750 |
| C3 (mg/dL) | 61.7 ± 69.0 | 66.3 ± 74.7 | 0.753 |
| C4 (mg/dL) | 14.2 ± 16.4 | 13.4 ± 15.3 | 0.812 |
| BNP (pg/mL) | 641.3 ± 785.3 | 780.3 ± 1454.9 | 0.736 |
ESR erythrocyte sedimentation rate, SF serum ferritin, PCT procalcitonin, CRP C-reactive protein, WBC white blood cell, ANC absolute neutrophil count, ALC absolute lymphocyte count, ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, GGT γ-glutamyl transferase, LDH lactate dehydrogenase, TG triglycerides, IL interleukin, TNF-α tumor necrosis factor-α, Ig immunoglobulin, BNP brain natriuretic peptide. #ALC < 1 × 109/L. *P < 0.05, **P < 0.01, ***P < 0.001
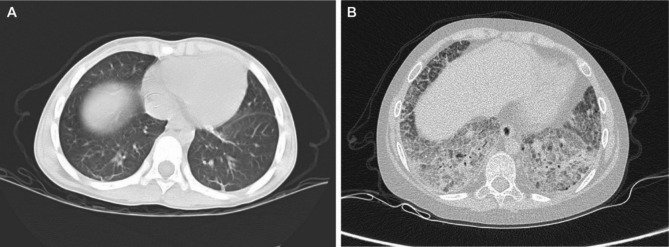
All 35 children with SJIA-ILD exhibited abnormal HRCT findings, including 24 cases of reticular patterns, 14 cases of consolidation, 12 cases of interlobular septal thickening, 10 cases of ground-glass opacification, 2 cases of honeycombing, and 2 cases of bronchial dilatation. 10 children underwent pulmonary function tests, including 8 showing decreased in forced vital capacity and 5 having decreased diffusing capacity of carbon monoxide. In Fig. 2, representative images of HRCT findings of ILD related SJIA are shown.
Fig. 2.
Representative HRCT images in patients with SJIA-ILD. A Subpleural linear shadow (early interstitial lung disease); B Crazy-paving sign, honeycomb lung (advanced interstitial lung disease)
Treatment and outcome
The median follow-up duration was 6.0 years (range 3.9 years to 9.5 years). 32 Patients (91.4%) received high-dose corticosteroids (≥ 1 mg/kg/day prednisone equivalent) in the SJIA with ILD group, which was significantly more than those in non-ILD group (P = 0.018). Eight patients (22.9%) with SJIA-ILD were treated with pulse methylprednisolone therapy. Cyclophosphamide (8.6% vs. 0, P = 0.007), leflunomide (28.6% vs. 11.3%, P = 0.010), and hydroxychloroquine (14.3% vs. 1.4%, P = 0.003) were significantly more frequently used in SJIA patients with ILD than in those without ILD. More ILD patients (54.3%) required combination therapy to control disease activity (P = 0.006). Trimethoprim-sulfamethoxazole (TMP-SMX) was used as a prophylactic treatment to prevent infections in patients underwent high-dose corticosteroid, especially accompanied by lymphopenia. In addition, 16 patients (45.7%) developed LD who were never exposed to bDMARDs, and 19 patients (54.3%) with SJIA-ILD were treated with bDMARDs, with tocilizumab (51.4%) being the most commonly used drug. However, only three patient receiving biologics (1 case with IL-6 blockade, 2 cases with TNF inhibitor) prior to ILD onset and none receiving IL-1 blockade. There was no significant difference in the use of biological agents between the ILD and non-ILD groups (P = 0.912) (Table 3).
Table 3.
Therapeutic intervention of patients with SJIA
| Treatment | ILD (n = 35) |
Non-ILD (n = 141) |
P value |
|---|---|---|---|
| Methotrexate | 25 (71.4) | 113 (80.1) | 0.262 |
| Sulfasalazine | 2 (5.7) | 11 (7.8) | 0.951 |
| Leflunomide | 10 (28.6) | 16 (11.3) | 0.010* |
| Thalidomide | 10 (28.6) | 28 (19.9) | 0.262 |
| Hydroxychloroquine | 5 (14.3) | 2 (1.4) | 0.003** |
| Cyclosporine | 12 (34.3) | 27 (19.1) | 0.054 |
| Tacrolimus | 1 (2.9) | 1 (0.7) | 0.359 |
| Cyclophosphamide | 3 (8.6) | 0 (0.0) | 0.007** |
| bDMARDs | 19 (54.3) | 78 (55.3) | 0.912 |
| Etanercept | 2 (5.7) | 12 (8.5) | 0.843 |
| Infliximab | 2 (5.7) | 5 (3.5) | 0.917 |
| Adalimumab | 2 (5.7) | 6 (4.3) | 1.000 |
| Tocilizumab | 18 (51.4) | 73 (51.8) | 0.971 |
| Combination therapy# | 19 (54.3) | 42 (29.8) | 0.006** |
| IVIG | 20 (57.1) | 63 (44.7) | 0.186 |
| High-dose corticosteroids## | 32 (91.4) | 102 (72.3) | 0.018* |
| Pulse therapy | 8 (22.9) | 29 (20.6) | 0.766 |
Values are presented as n (%). bDMARDs biological disease-modifying anti-rheumatic drugs, IVIG Intravenous immunoglobulin. #Patient received either more than two conventional DMARDs or one conventional DMARD combined with one bDMARD. ##Prednisone equivalent ≥ 1 mg/kg/day. *P < 0.05, **P < 0.01
In terms of treatment response, the proportion of patients achieving treatment Goal one (48.6% vs. 70.9%, P = 0.012) and treatment Goal three (45.7% vs. 70.2%, P = 0.006) were significantly lower in ILD patients compared to the control group. It was more challenging to achieve CR (54.3% vs. 78.0%, P = 0.005) and relatively higher rates of NR (17.1% vs. 3.5%, P = 0.010) in ILD patients during disease follow-up. Two cases of ILD pediatric patients resulted in death, both due to secondary severe lung infections and respiratory failure. No death was observed in the non-ILD group. It was interesting that there was no significant difference in disease relapse rates between the two groups (P = 0.271) (Table 4).
Table 4.
Outcome and prognosis of patients with SJIA
| ILD (n = 35) |
Non-ILD (n = 141) |
P value | |
|---|---|---|---|
| Goal one | 17 (48.6) | 100 (70.9) | 0.012* |
| Goal two | 28 (80.0) | 124 (87.9) | 0.342 |
| Goal three | 16 (45.7) | 99 (70.2) | 0.006** |
| Complete remission | 19 (54.3) | 110 (78.0) | 0.005** |
| Partial remission | 10 (28.6) | 26 (18.4) | 0.184 |
| No remission | 6 (17.1) | 5 (3.5) | 0.010* |
| Death | 2 (5.7) | 0 (0.0) | 0.039* |
| Relapse | 18 (51.4) | 58 (41.1) | 0.271 |
| Once | 7 (20.0) | 33 (23.4) | 0.667 |
| Twice | 4 (11.4) | 10 (7.1) | 0.617 |
| More than twice | 7 (20.0) | 15 (10.6) | 0.225 |
Values are presented as n (%). *P < 0.05, **P < 0.01
Discussion
ILD is a life-threatening and increasing recognized complication in children with SJIA [8]. A screening algorithm recommended pulmonary screening for newly diagnosed patients with SJIA with “red flag features” due to increased risk for LD. These “red flag features” included baseline characteristics (young age of SJIA onset, HLA type, high disease activity), respiratory symptoms and features of drug hypersensitivity-like reactions [16]. The incidence of ILD in children with SJIA in this two-center study was 19.9%, consistent with 16% incidence rate of ILD in our previous single-center series [17]. Regardless, these were much higher than the rates reported in prior studies in other countries [4, 7]. The reason for this difference may be that all patients in our cohort underwent HRCT at the diagnosis, and we also found that some patients developed ILD during the course of the disease rather than at the onset. Among them, 4 cases were diagnosed through HRCT due to the lack of significant respiratory symptoms and X-ray findings. The relative low rate of clinical inactive disease due to under-use of biologics may be another potential reason.
Approximately 85.7% of patients with SJIA-ILD presented with arthritis at the onset of the disease in this study. Notably, the presence of axial joint involvement like the cervical spine and large joint involvement like the hip, known as poor prognostic risk factors, was more frequently observed in the ILD group. Laboratory examinations indicated that patients diagnosed with SJIA-ILD exhibited a marked inflammatory response with higher CRP, WBC, ANC, and ESR values and were more likely to exhibit hyperferritinemia and lymphopenia than those without ILD. These clinical features suggested higher disease activity and poor prognostic factors. Patients with lymphopenia were prone to secondary infections. Therefore, we adopted TMP-SMX prophylaxis in reducing the incidence of pneumocystis pneumonia with a favorable safety profile for these patients, consistent with other reports [18, 19].
Systemic juvenile idiopathic arthritis stands apart from other forms of JIA due to its propensity for life-threatening complications. A prime example of such a complication is MAS, a severe cytokine storm syndrome characterized by rampant systemic inflammation, profound hyperferritinemia, coagulopathy, and multiorgan dysfunction. In our study, about 40% of patients with ILD had at least one episode of MAS, significantly higher than that in the non-ILD group, as previously reported [8, 20]. Among these, 28.6% experienced more than two episodes of MAS. Furthermore, patients with SJIA-MAS exhibited a heightened susceptibility to ILD compared with those without MAS in a prior study [4]. Recent investigations had underscored the pivotal role of interferon-gamma (IFN-γ) in the pathogenesis of MAS. Promisingly, preliminary clinical trials evaluating emapalumab, an anti-IFN-γ monoclonal antibody, had yielded encouraging results. However, the reasons for the close association between SJIA-ILD and MAS were yet unclear. Gao et al. [21] discovered the crucial role of IFN-γ in triggering lung inflammation associated with MAS in a mouse model. Indeed, some patients with SJIA-ILD displayed elevated levels of IL-18 and IFN-γ-induced protein chemokine 9 (CXCL9), both specific markers of MAS [22, 23]. Moreover, our study revealed that more than 75% of patients with ILD exhibited SF level exceeding 684 ng/mL, including some cases of subclinical MAS. Therefore, we recommend every patient with SJIA-ILD should serve as a potentially crucial indicator for early screening of MAS.
In this study, only one patient received IL-6 blockade prior to SJIA-ILD onset and none received IL-1 blockade. There was no significant difference in the use of biologics between the ILD and non-ILD groups. The lower usage rate of biologics in our cohort was due to the fact that IL-6 antagonist was not available in China until the end of 2013 and IL-1 antagonist was still not available so far. Additionally, some children and their family could not afford the cost of biologics and had to rely on glucocorticoids or combined DMARDs treatment. A global case study involving 41 patients with SJIA-LD revealed some of which were linked to previous treatments with biological agents [6]. Additionally, patients with SJIA-ILD were reported with frequent occurrences of adverse reactions to biologics, such as delayed-type hypersensitivity or drug reactions with eosinophilia and systemic symptoms (DRESS-like reactions) [6, 8]. DRESS-like reactions typically present with fever, rash, lymphadenopathy, eosinophilia, atypical lymphocytosis and organ involvement [24, 25]. Recent studies have also connected these DRESS-like characteristics to the presence of the HLA-DRB1*15 alleles. Saper et al. [25] had associated these drug allergic reactions with the presence of HLA-DRB1*15 alleles. Interestingly, when comparing patients who stopped using IL-1/IL-6 antagonist permanently to those who maintained their treatment, there were no significant differences observed in terms of mortality rates or Physician Global Assessment of Lung Disease Activity (PGALD) scores [6]. However, DRESS-like reactions had not been observed in our cohort, and whether this was related to ethnic differences remains to be confirmed by further research.
This study assessed the differences in treatment targets between ILD and non-ILD groups based on the short-term (Goal one), the mid-term (Goal two), and the long-time target (Goal three) proposed by Hinze et al. in 2018 [26]. The ILD group required a prolonged time to control inflammation, and the proportion of children achieving inactive status within one year was lower than that of the control group. Lower remission rates indicated a poor prognosis, and 17% of patients with ILD remained in an unremitted state by the end of follow-up, including two cases of death. The mortality rate in our cohort was 5.7%, which was substantially lower than previous estimates ranging from 58–68% [5, 7]. Recent data showed patients with SJIA-ILD had frequent hypoxia and requirement for respiratory support with lower mortality than previously reported [6], similar to the results of our study. Intensive treatment in the early stages of the disease to control disease activity as early as possible may be beneficial in reducing the development of ILD. Considering the variety of treatment approaches within our cohort, it was not feasible to assess the efficacy of any single therapy. The diversity in treatment approaches highlighted the critical need for targeted clinical trials.
Our study has several limitations. First, as a retrospective analysis, it may introduce statistical biases, and treatment strategies were not standardized. Second, the cohort was from only two centers, with a relatively small sample size, highlighting the need for larger-scale data from domestic multicenter studies. Third, while we summarized clinical characteristics and prognosis, we did not investigate the pathogenesis of ILD, including cytokine profiling such as IL-18, CXCL9, MMP7 and HLA type [8, 27, 28]. Further prospective studies are essential to better understand the underlying mechanisms and develop targeted interventions to improve patient outcomes.
Conclusion
Patients with SJIA-ILD demonstrated a more pronounced inflammatory response and showed a greater tendency for involvement of major joints like the hip and axial joints such as the cervical spine. They were also more susceptible to developing lymphopenia and MAS, signaling a comparatively poor prognosis with a heightened risk of mortality. Managing inflammation in these patients required extended periods and more intensive treatment strategies to reach inactive status. The suboptimal rate of complete remission observed in this study underscores the need for further investigation into treatment protocols for these patients.
Acknowledgements
We thank all our patients and their parents for their contribution to our research.
Abbreviations
- ACR
American College of Rheumatology
- ANC
Absolute neutrophil count
- bDMARDs
Biological disease-modifying anti-rheumatic drugs
- ChILD
Childhood interstitial lung disease
- CID
Clinical inactive disease
- CR
Complete remission
- CRP
C-reactive protein
- CXCL9
Chemokine 9
- ESR
Erythrocyte sedimentation rate
- HRCT
High-resolution computed tomography
- IFN-γ
Interferon-gamma
- IL
Interleukin
- ILAR
The International League of Rheumatology Alliance
- ILD
Interstitial lung disease
- JADAS-27
Juvenile Arthritis Disease Activity Score-27
- MAS
Macrophage activation syndrome
- NR
No remission
- PGALD
Physician Global Assessment of Lung Disease Activity
- PR
Partial remission
- SD
Standard deviation
- SF
Serum ferritin
- SJIA
Systemic juvenile idiopathic arthritis
- TMP-SMX
Trimethoprim-sulfamethoxazole
- VAS
Visual analog scale
- WBC
White blood cell
Author contributions
WTZ, JXY, and LZQ wrote the initial draft of the manuscript. LZQ, KKY, and YYSG contributed to data collection. WTZ, JXY, and XHY. contributed to data analysis and interpretation. HGY and WJZ contributed to study design, edit and performed the final manuscript review. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of Zhejiang Province (LY23H100002), Zhejiang Provincial Medical Science and Technology Project (2022KY904), and Wenzhou Basic Scientific Research Project (Y20210260).
Data availability
The datasets generated and analyzed during the current study are not publicly available because the dataset will be further studied to publish other works but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the Second Affiliated Hospital of Wenzhou Medical University (No. 2021-K-258-02) and the Children’s Hospital of Nanjing Medical University (No. 20150629-1).
Consent for publication
NA.
Competing interests
No competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenting Zhan, Jinxiang Yang and Lingzhi Qiu contributed equally to this work.
Contributor Information
Haiguo Yu, Email: haiguo_yu@njmu.edu.cn.
Wenjie Zheng, Email: wzwjzheng@sina.com.
References
- 1.Kumar S. Systemic juvenile idiopathic arthritis: diagnosis and management. Indian J Pediatr. 2016;83(4):322–7. [DOI] [PubMed] [Google Scholar]
- 2.Martini A. Systemic juvenile idiopathic arthritis. Autoimmun Rev. 2012;12(1):56–9. [DOI] [PubMed]
- 3.Minoia F, Davì S, Horne A, Demirkaya E, Bovis F, Li C, et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, Multicenter Study of 362 patients. Arthritis Rheumatol. 2014;66(11):3160–9. [DOI] [PubMed] [Google Scholar]
- 4.Schulert GS, Yasin S, Carey B, Chalk C, Do T, Schapiro AH, et al. Systemic juvenile idiopathic arthritis–Associated Lung Disease: characterization and risk factors. Arthritis Rheumatol. 2019;71(11):1943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saper VE, Chen G, Deutsch GH, Guillerman RP, Birgmeier J, Jagadeesh K, et al. Emergent high fatality lung disease in systemic juvenile arthritis. Ann Rheum Dis. 2019;78(12):1722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Sompii-Montgomery L, Patti J, Pickering A, Yasin S, Do T, et al. Disease Course, treatments, and outcomes of children with systemic juvenile idiopathic arthritis–Associated Lung Disease. Arthritis Care Res (Hoboken). 2023;76(3):328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura Y, Weiss JE, Haroldson KL, Lee T, Punaro M, Oliveira S, et al. Pulmonary hypertension and other potentially fatal pulmonary complications in systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2013;65(5):745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wobma H, Arvila SR, Taylor ML, Lam KP, Ohashi M, Gebhart C, et al. Incidence and risk factors for Eosinophilia and Lung Disease in Biologic-exposed children with systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2023;75(10):2063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 10.Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, Pediatric Rheumatology International trials Organization International Consensus. J Rheumatol. 2019;46(2):190–7. [DOI] [PubMed] [Google Scholar]
- 11.Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A, et al. 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 2016;68(3):566–76. [DOI] [PubMed] [Google Scholar]
- 12.Semple T, Winant AJ, Lee EY. Childhood interstitial lung disease: imaging guidelines and recommendations. Radiol Clin North Am. 2022;60(1):83–111. [DOI] [PubMed] [Google Scholar]
- 13.Consolaro A, Ruperto N, Bazso A, Pistorio A, Magni-Manzoni S, Filocamo G, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61(5):658–66. [DOI] [PubMed] [Google Scholar]
- 14.Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2011;63(7):929–36. [DOI] [PubMed] [Google Scholar]
- 15.Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40(7):1202–9. [DOI] [PubMed] [Google Scholar]
- 16.Wobma H, Bachrach R, Farrell J, Chang MH, Day-Lewis M, Dedeoglu F, et al. Development of a screening algorithm for lung disease in systemic juvenile idiopathic arthritis. ACR Open Rheumatol. 2023;5(10):556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Xie X, Yang K, Zheng W. Clinical analysis of systemic onset juvenile idiopathic arthritis complicated with interstitial lung disease. Chin J Rheumatol. 2021;25(8):521–4. [Google Scholar]
- 18.Park JW, Curtis JR, Kim MJ, Lee H, Song YW, Lee EB. Pneumocystis pneumonia in patients with rheumatic diseases receiving prolonged, non-high-dose steroids-clinical implication of primary prophylaxis using trimethoprim-sulfamethoxazole. Arthritis Res Ther. 2019;21(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis. 2018;77(5):644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Towe C, Grom AA, Schulert GS. Diagnosis and management of the systemic juvenile idiopathic arthritis patient with emerging Lung Disease. Paediatr Drugs. 2023;25(6):649–58. [DOI] [PubMed] [Google Scholar]
- 21.Gao DK, Salomonis N, Henderlight M, Woods C, Thakkar K, Grom AA, et al. IFN-γ is essential for alveolar macrophage–driven pulmonary inflammation in macrophage activation syndrome. JCI Insight. 2021;6(17):e147593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prencipe G, Bracaglia C, De Benedetti F. Interleukin-18 in pediatric rheumatic diseases. Curr Opin Rheumatol. 2019;31(5):421–7. [DOI] [PubMed] [Google Scholar]
- 23.Verweyen EL, Thakkar K, Dhakal S, Baker E, Chetal K, Schnell D, et al. Population-level single-cell genomics reveals conserved gene programs in systemic juvenile idiopathic arthritis. J Clin Invest. 2023;133(22):e166741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169(5):1071–80. [DOI] [PubMed] [Google Scholar]
- 25.Saper VE, Ombrello MJ, Tremoulet AH, Montero-Martin G, Prahalad S, Canna S, et al. Severe delayed hypersensitivity reactions to IL-1 and IL-6 inhibitors link to common HLA-DRB1*15 alleles. Ann Rheum Dis. 2022;81(3):406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinze CH, Holzinger D, Lainka E, Haas J-P, Speth F, Kallinich T, et al. Practice and consensus-based strategies in diagnosing and managing systemic juvenile idiopathic arthritis in Germany. Pediatr Rheumatol Online J. 2018;16(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5(4):e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrongari D, Di Filippo P, Misticoni F, Basile G, Di Pillo S, Chiarelli F, Attanasi M. Lung involvement in systemic juvenile idiopathic arthritis: a narrative review. Diagnostics (Basel). 2022;12(12):3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available because the dataset will be further studied to publish other works but are available from the corresponding author on reasonable request.