Abstract
Undifferentiated pleomorphic sarcomas (UPS) represent a prevalent and aggressive subtype of soft tissue sarcomas (STS) in adults. Despite advancements in loco regional treatments, many patients with high grade STS, including UPS, develop metastatic disease. Neoadjuvant chemotherapy is a standard approach to mitigate this risk, but response variability necessitates refined patient selection strategies. This study investigated the correlation between UPS microenvironment and neoadjuvant chemotherapy response in resectable UPS. The NEOSARCOMICS study (NCT02789384) enrolled patients with resectable STS from six sarcoma centers in France. Patients received anthracycline based chemotherapy, followed by surgery. Histological response, gene expression profiling, and multiplex immunohistofluorescence were performed on baseline and post treatment tumor samples. Plasma proteomics was analyzed to identify biomarkers. Good responders to neoadjuvant chemotherapy showed enrichment in genes related to stemness and cell cycle regulation, while poor responders exhibited immune related gene enrichment. Proteomic profiling revealed immune pathway activation and downregulation of cell cycle pathways in non responders. Despite being associated with a good prognosis, high immune infiltration, particularly of CD8 + T cells and CD20 + B cells, predicts a poor response to neoadjuvant chemotherapy in UPS, suggesting the need for alternative therapeutic strategies for patients with inflamed UPS.Ongoing clinical trials are exploring the efficacy of combining chemotherapy with immune checkpoint inhibitors to improve outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13045-024-01614-w.
Keywords: Soft tissue sarcoma
To the Editor
Undifferentiated pleomorphic sarcomas (UPS) are a prevalent and aggressive subtype of soft tissue sarcomas (STS) in adults [1]. We have previously shown that there are two distinct UPS subgroups: immune high, with increased immune infiltrates and upregulated immune checkpoints, associated with lower metastatic relapse and better survival; and immune low, characterized by more gene copy number alterations, particularly in tumor suppressor genes, and a poorer prognosis [2].
Neoadjuvant chemotherapy is often used to reduce the risk of metastatic relapse in patients with high-grade UPS [3], though its variable efficacy necessitates better patient selection strategies. Studies in epithelial tumors have shown a link between the tumor microenvironment and chemotherapy response [4]. We hypothesize that UPS response to neoadjuvant chemotherapy is influenced by immune cell composition.
To confirm the prognostic value of the immune classification of UPS we previously identified [2], we first investigated whether the amount of tumor infiltrating immune cells influenced the risk of metastatic relapse and death in a cohort of 47 patients with UPS who underwent surgery for localized disease. Tissue microarrays of UPS samples were stained with the multiplex IHF panel combining CD8, CD14, CD20, CD45, CD68, cMAF and DAPI markers (Supplementary Methods, Supplementary Fig. 1A). The patients’ characteristics are described in Supplementary Table 1. We observed that patients with high SARCULATOR total score, e.g. low survival probabilities, were less infiltrated in immune cells and notably in CD8 + cells and M1 macrophages (CD68+/cMAF cells) (Supplementary Fig. 1B). Similarly, patients with high levels of CD20+, CD8+, CD14 + cells or M2 macrophages (CD68+/cMAF + cells) tend to have a better overall survival than UPS patients with lower infiltration (Supplementary Fig. 1C).
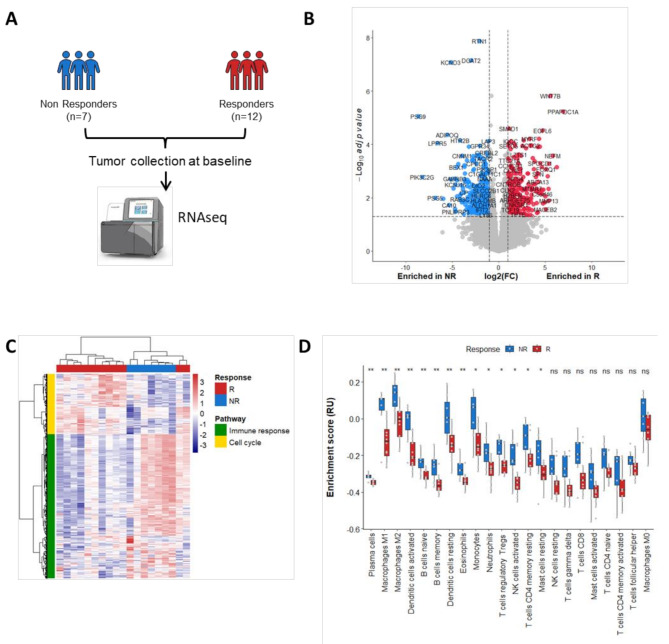
Then to decipher the impact of UPS microenvironment on response to neoadjuvant chemotherapy, we first performed gene expression profiling of baseline samples from 24 patients with resectable UPS who were treated with anthracycline based neoadjuvant chemotherapy and enrolled in the NEOSARCOMICS study (Supplementary Table 2, Supplementary Methods, Fig. 1A). Twelve patients had a good histological response after central blinded pathological assessment. The examination of differential gene expression between good responders and poor responders to neoadjuvant chemotherapy unveiled 1058 genes (Fig. 1B Supplementary Table 3).
Fig. 1.
Response to neoadjuvant chemotherapy correlates with high proliferation and low immune infiltration phenotype. (A) Workflow of RNAseq experiment performed on baseline tumor samples from UPS patients treated with neoadjuvant chemotherapy. (B) Volcano plot representation of genes differentially expressed between responder (R) and non responder (NR) patients. (C) Heatmap visualization of Gene Ontology biological process enrichment scores. (D) Boxplot representation of immune cells estimation (CIBERSORT) according to response to neoadjuvant chemotherapy. P values were calculated using Wilcoxon tests
The good responders group showed significant enrichment in genes related to stemness, cell cycle regulation, and oncogenesis (Fig. 1C, Supplementary Table 3). This included key genes like LHX8, involved in stem cell fate [5]; CCNE1, CDC25A, and CDK2, which regulate the G1/S cell cycle transition [6]; DNA polymerase genes (POLE, POLM, POLD1); and FGFR2, previously identified in the immune low UPS subgroup [2]. In contrast, poor responders exhibited enrichment in genes related to immune response pathways, such as type I IFN signaling and myeloid and lymphocyte activation, suggesting a strong immune presence in the tumor microenvironment (Fig. 1C, Supplementary Table 3). CIBERSORT analysis further revealed that poor responders were highly enriched in immune cells (Fig. 1D).
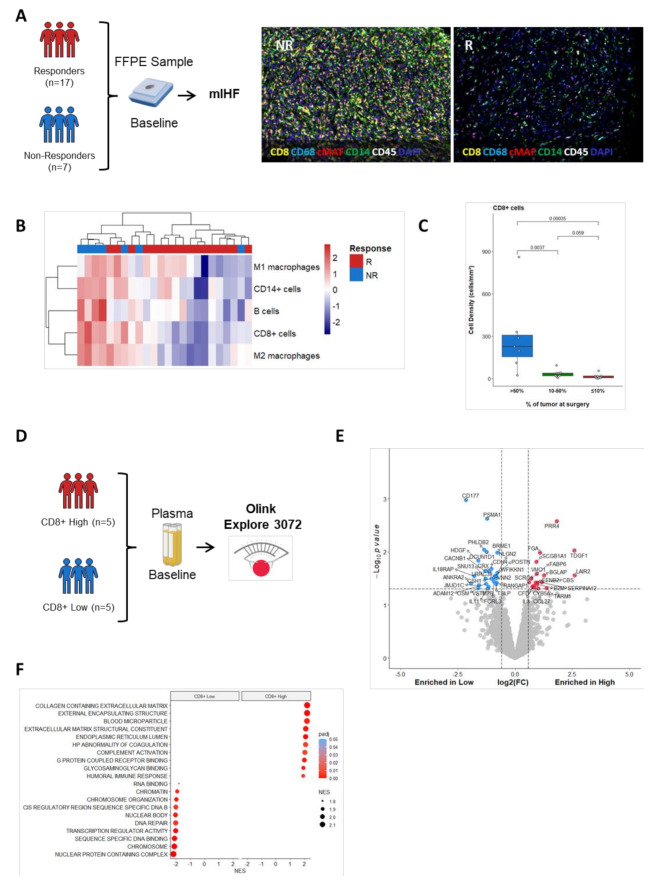
To visualize the difference in immune cell abundances between responders and non-responders and confirm gene expression data, baseline tumor samples were stained with the multiplex IHF panel CD8 / CD14 / CD20 / CD45 / CD68 / cMAF / DAPI (Supplementary Methods, Fig. 2A). Quantification of immune cell density confirmed that baseline samples from patients with a poor response to neoadjuvant chemotherapy tended to be highly infiltrated by immune cells (Fig. 2B and C). Analysis of plasma proteins (Supplementary Methods, Fig. 2D) differentially expressed at baseline between CD8 + High and Low UPS patients highlighted the upregulation of cell cycle pathways in patients with low immune infiltration (Fig. 2E F).
Fig. 2.
Poor response to neoadjuvant chemotherapy is associated with baseline immune infiltrates in UPS patients. (A) Workflow of multiplex IHF validation experiment (left). Representative images of tumor FFPE section of non responder (NR) and responder (R) patients stained with the multiplex panel CD8 / CD14 / CD20 / CD45 / CD68 / cMAF / DAPI. (B) Heatmap visualization of cell densities of indicated immune cell populations in UPS patients. (C) Boxplot representation of CD8 + cell densities according to percentage of tumors cell at surgery. P values were calculated using Wilcoxon tests. (D) Workflow of plasma proteomic analysis using Olink Explore 3072. (E) Volcano plot representation of protein differentially secreted at baseline between CD8 + high and low UPS patients. (F) Bubble plot of Gene Ontology terms enrichment
To assess the impact of neoadjuvant chemotherapy on the tumor microenvironment, we analyzed gene expression profiles from paired pretreatment biopsy and surgical specimens in responders (n = 3) and non-responders (n = 5) (Supplementary Fig. 2A). Differentially expressed genes at surgery varied significantly between the two groups (Supplementary Fig. 2B). Hallmark gene signature analysis showed that allograft rejection was elevated in responders, while TNFα and Wnt/βcatenin signaling were specific to non-responders (Supplementary Fig. 2C). Deconvolution analysis revealed increased infiltration of cytotoxic CD8 + T cells and CD20 + B cells in responders, but not in non-responders (Supplementary Fig. 2D). Additionally, plasma proteomics linked a good response to higher levels of CD5L and lower levels of GDF 15 (Supplementary Fig. 3).
While high immune infiltration of UPS correlates with better survival, it predicts poor pathological response to chemotherapy, emphasizing the complex role of the tumor microenvironment. A key factor may be the presence of M2 macrophages, enriched in non-responders, which are linked to chemoresistance by suppressing T cell function and promoting tumor survival [7, 8]. Regulatory T cells (Tregs) were also enriched in poor responders, mirroring findings in breast cancer, where Tregs are linked to poor chemotherapy responses [9, 10].
Although this study focused on immune infiltration, the stromal and extracellular matrix (ECM) components of the tumor microenvironment also likely affect chemotherapy response by acting as physical barriers to drug delivery [11]. Conversely, the immune low group may respond better to chemotherapy, with an enrichment of genes involved in cell cycle regulation and oncogenesis [2].
Our results could help stratify UPS patients by immune status for more personalized treatments. Our findings suggest that standard chemotherapy may not be optimal for immune high UPS patients. Combining chemotherapy with immune checkpoint inhibitors or targeting tumor-associated macrophages could offer better outcomes [12]. Ongoing studies, such as NCT04968106, are exploring chemoimmunotherapy combinations in high-grade UPS, with histological response as a primary endpoint, potentially advancing our understanding of immune-tumor interactions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- cMAF
Musculoaponeurotic Fibrosarcoma Oncogene Homolog
- CD
Cluster of Differentiation
- DAPI
4’,6 Diamidino 2 Phenylindole
- DMFS
Distant Metastases Free Survival
- DNA
Deoxyribonucleic Acid
- ECOG
Eastern Cooperative Oncology Group
- ECM
Extracellular Matrix
- EORTC STBSG
European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group
- GDF 15
Growth Differentiation Factor 15
- HRP
Horseradish Peroxidase
- IHC
Immunohistochemistry
- IFN
Interferon
- mTOR
Mechanistic Target of Rapamycin
- NCT
National Clinical Trial
- NGS
Next Generation Sequencing
- NPX
Normalized Protein Expression
- OS
Overall Survival
- PCR
Polymerase Chain Reaction
- PEA
Proximity Extension Assay
- PI3K
Phosphoinositide 3 Kinase
- RNA
Ribonucleic Acid
- SARCULATOR
Sarcoma Calculator
- STS
Soft Tissue Sarcomas
- TME
Tumor Microenvironment
- TNFα
Tumor Necrosis Factor Alpha
- Tregs
Regulatory T Cells
- TSA
Tyramide Signal Amplification
- UPS
Undifferentiated Pleomorphic Sarcomas
- Wnt
Wingless related integration site
Author contributions
“AI, AB, conceived and designed the study. LV and JMC performed the histological analyses. MT, MSC and FP. JPG, NEG and JV performed the statistical analyses. All authors collected and assembled data. AI, AB, NEG and JPG developed the tables and figures. AI, AB, NEG and JPG conducted the literature search and wrote the manuscript. All authors were involved in the critical review of the manuscript and approved the final version.”
Funding
This study was supported by RHU CONDOR Institut National du Cancer and Association pour la Recherche contre le Cancer.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
This study was approved by a Central Institutional Review Board (Comité de Protection des Personnes Sud Est II, Lyon, France), according to good clinical practices and applicable laws and regulations. All methods were performed in accordance with the relevant guidelines and regulations. All patients provided written informed consent.
Competing interests
AB and JPG: Employees of Immusmol/Explicyte. AI: Received research grants from Astra Zeneca, Bayer, BMS, Chugai, Merck, MSD, Pharmamar, Novartis, Roche, and received personal fees from BMS, MSD, Merck, Roche, Epizyme, Bayer, Lilly, Roche, and Springworks. The other authors have nothing to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Makris EA, Tran TB, Delitto DJ, Lee B, Ethun CG, Grignol V, Harrison Howard J, Bedi M, Clark Gamblin T, Tseng J, Roggin KK, Chouliaras K, Votanopoulos K, Cullinan D, Fields RC, Cardona K, Poultsides G, Kirane A. Natural history of undifferentiated pleomorphic sarcoma: experience from the US Sarcoma Collaborative. J Surg Oncol. 2024;129(7):13541363. [DOI] [PubMed] [Google Scholar]
- 2.Toulmonde M, Lucchesi C, Verbeke S, Crombe A, Adam J, Geneste D, Chaire V, Laroche Clary A, Perret R, Bertucci F, Bertolo F, Bianchini L, Dadone Montaudie B, Hembrough T, Sweet S, Kim YJ, Cecchi F, Le Loarer F, Italiano A. High throughput profiling of undifferentiated pleomorphic sarcomas identifies two main subgroups with distinct immune profile, clinical outcome and sensitivity to targeted therapies. EBioMedicine. 2020;62:103131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brennan B, Brodowicz T, Buonadonna A, De Álava E, Del Muro XG, Dufresne A, Eriksson M, Fagioli F, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hecker Nolting S, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kager L, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez Pousa A, Martin Broto J, Merimsky O, Messiou C, Mir O, Montemurro M, Morland B, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss S, Sundby Hall K, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Casali PG, Stacchiotti S. ESMO Guidelines Committee, EURACAN and GENTURIS. Electronic address: clinicalguidelines@esmo.org. Soft tissue and visceral sarcomas: ESMO EURACAN GENTURIS Clinical Practice guidelines for diagnosis, treatment and follow up☆. Ann Oncol. 2021;32(11):13481365. [DOI] [PubMed] [Google Scholar]
- 4.Derouane F, van Marcke C, Berlière M, Gerday A, Fellah L, Leconte I, Van Bockstal MR, Galant C, Corbet C, Duhoux FP. Predictive biomarkers of response to neoadjuvant chemotherapy in breast Cancer: current and future perspectives for Precision Medicine. Cancers (Basel). 2022;14(16):3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Yang G, Chen M, He L, Xiang L, Ricupero C, Mao JJ, Ling J. Lhx6 and Lhx8: cell fate regulators and beyond. FASEB J. 2015;29(10):408391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagundes R, Teixeira LK. Cyclin E/CDK2: DNA replication, replication stress and genomic instability. Front Cell Dev Biol. 2021;9:774845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye JH, Wang XH, Shi JJ, Yin X, Chen C, Chen Y, Wu HY, Jiong S, Sun Q, Zhang M, Shi XB, Zhou GR, Hassan S, Feng JF, Xu XY, Zhang WJ. Tumor associated macrophages are associated with response to neoadjuvant chemotherapy and poor outcomes in patients with triple negative breast cancer. J Cancer. 2021;12(10):28862892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y, et al. High infiltration of tumor associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111(6):752–9. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Wang X, Deng Y, Yu X, Wang H, Li Z. Research Progress on the Role of Regulatory T Cell in Tumor Microenvironment in the treatment of breast Cancer. Front Oncol. 2021;11:766248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladoire S, Arnould L, Apetoh L, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor infiltrating Foxp3 + regulatory T cells. Clin Cancer Res. 2008;14:2413–20. [DOI] [PubMed] [Google Scholar]
- 11.Druzhkova I, Nikonova E, Ignatova N, Koryakina I, Zyuzin M, Mozherov A, Kozlov D, Krylov D, Kuznetsova D, Lisitsa U, Shcheslavskiy V, Shirshin EA, Zagaynova E, Shirmanova M. Effect of collagen matrix on Doxorubicin distribution and Cancer cells’ response to treatment in 3D Tumor Model. Cancers (Basel). 2022;14(22):5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molgora M, Esaulova E, Vermi W, Hou J, Chen Y, Luo J, Brioschi S, Bugatti M, Omodei AS, Ricci B, Fronick C, Panda SK, Takeuchi Y, Gubin MM, Faccio R, Cella M, Gilfillan S, Unanue ER, Artyomov MN, Schreiber RD, Colonna M. TREM2 Modulation Remodels the Tumor Myeloid Landscape Enhancing Anti PD 1 Immunotherapy. Cell. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.