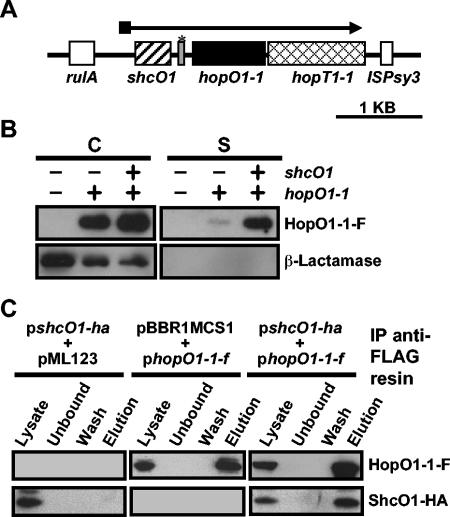
FIG. 1.
ShcO1 facilitates the type III secretion of HopO1-1 and interacts with HopO1-1 in vivo. (A) Genetic organization of the HopO1-1 operon. The operon is located on plasmid pDC3000A in DC3000. The TTC gene shcO1 is depicted with a striped box. The hopO1-1 and hopT1-1 genes are depicted with a black box and a hatched box, respectively. The open boxes show the genes flanking the hopO1-1 operon. The gray box marked with an asterisk between shcO1 and hopO1-1 represents a gene fragment which is homologous to hopS1, a gene shown in Fig. 2A. (B) Secretion of HopO1-1 in culture via the DC3000 TTSS is enhanced in the presence of the ShcO1 TTC. Constructs containing hopO1-1-flag (pLN142) or shcO1/hopO1-1-flag (pLN411) were electroporated into the DC3000 hopO1-1 operon deletion mutant UNL137, which also carried pCPP2318 encoding β-lactamase lacking its export signal. Cultures of the above strains were grown in hrp-inducing fructose medium and separated into cell (C) and supernatant (S) fractions. Proteins were resolved with SDS-PAGE and transferred to polyvinylidene difluoride membranes. HopO1-1-F and β-lactamase were detected with anti-FLAG and anti-β-lactamase antibodies, respectively. (C) ShcO1 coimmunoprecipitates with HopO1-1. DC3000(pLN142, pLN898) cultures, which expressed ShcO1-HA and HopO1-1-F, were sonicated, and soluble protein samples were obtained by centrifugation. Soluble proteins were mixed with anti-FLAG resin to immunoprecipitate (IP) HopO1-1-F as described in Materials and Methods. DC3000(pLN898, pML123) and DC3000(pBBR1MCS1, pLN142) were used as vector controls. Fractions representing the total soluble protein (Lysate), total soluble protein after treatment with resin (Unbound), the final wash (Wash), and the proteins that were eluted from the resin (Elution) were subjected to SDS-PAGE and immunoblotted. ShcO1-HA and HopO1-1-F were detected with anti-HA and anti-FLAG antibodies, respectively.