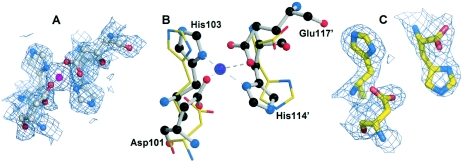
FIG. 4.
Comparison of a filled and an empty type 2 metal binding site. (A and C) Close-ups of the type 2 metal binding sites (shown is the 2Fo-Fc simulated annealing omit electron density map contoured at the 1σ level) with and without zinc (purple sphere), respectively. (B) Superposition of the empty (in dimerb) and filled (in dimera) zinc sites shows how the backbone and side chain atoms of the histidine and aspartate pairs differ. His-114 is the last residue that can be seen in dimerb. The filled site is shown as a ball-and-stick diagram, and the empty site as bonds.