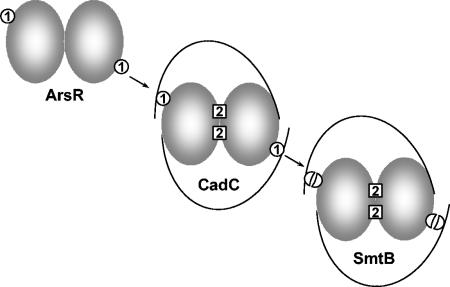
FIG. 6.
Model for the evolutionary history of type 1 and type 2 metal binding sites. The type 1 metal binding sites in ArsR are proposed to be the most ancient, because each site requires only two residues (Cys-32 and Cys-34) from a single subunit of the homodimer. In CadC, the equivalents of these two cysteine residues, Cys-58 and Cys-60, form more-complicated type 1 sites with residues Cys-7′ and Cys-11′ from the other subunit. CadC also has type 2 sites composed of residues Asp-101 and His-103 from one subunit and residues His-114′ and Glu-117′ from the other subunit. The equivalent type 2 sites in SmtB are composed of residues Asp-104 and His-106 from one subunit and His-117′ and Glu-120′ from the other subunit. SmtB also has residue Cys-14, which corresponds to either CadC residue Cys-7 or CadC residue Cys-11, and residue Cys-61′, which corresponds to CadC residue Cys-58′ and may represent a degenerate type 1 site.