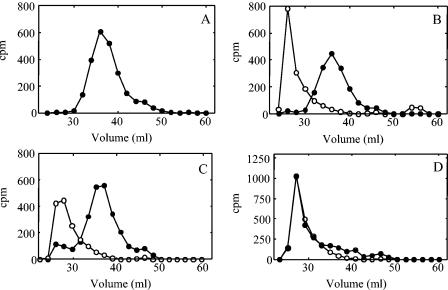
FIG. 8.
Reinitiation of polysaccharide synthesis following ejection of the nascent polymer. [14C]GlcUA-labeled polysaccharide-enyzme complexes were prepared in a 500-μl reaction mixture consisting of 100 mM HEPES (pH 7.5), 10 mM MnCl2, 100 μM UDP-Glc, 100 μM UDP-[14C]GlcUA (27 mCi/mmol), and JD614 membranes (1.5 mg of protein). The reaction was incubated for 5 min at 35°C and stopped by placing it on ice. The volume of the reaction was brought to 2 ml with wash buffer (100 mM HEPES [pH 7.5] containing 10% glycerol), and the membranes were washed four times. (A) An aliquot of the 14C-labeled product was chromatographed on a Sephacryl S-500 column. Aliquots of the labeled complex were incubated for 10 min at 35°C in 100-μl-volume reactions containing 100 mM HEPES (pH 7.5), 10 mM MnCl2, and either (B) 100 μM UDP-Glc, (C) 100 μM UDP-GlcUA, or (D) no UDP-sugars. Membranes were sedimented by centrifugation and washed once as described above. The volume was then brought to 100 μl so that the reaction contained 100 mM HEPES (pH 7.5), 10 mM MnCl2, 20 μM UDP-[3H]Glc (20 μCi/mmol), and 20 μM UDP-GlcUA. The reactions were incubated for an additional 5 min at 35°C and terminated by placing them on ice and adding 200 μl of ice-cold wash buffer. The membranes were sedimented by centrifugation, washed, and analyzed by chromatography on a Sephacryl S-500 column. 14C (•) and 3H (○) levels were measured in the even-numbered fractions. The values compensate for a 17% overlap of 14C and a 0.2% overlap of 3H in the corresponding channels.