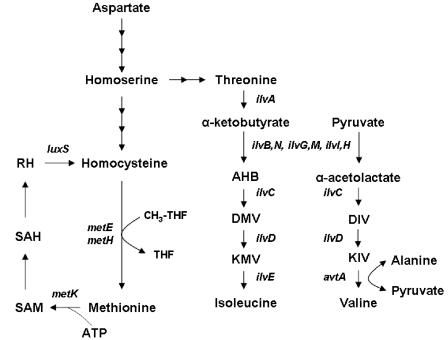
FIG. 1.
Role of Hcy in methionine biosynthesis in E. coli. The final step in the biosynthesis of methionine from homoserine involves the transfer of a methyl group from the methyl tetrahydrofolate (CH3-THF) to Hcy. The single carbon unit is derived from serine. The reaction is catalyzed by either of two enzymes, the vitamin B12-dependent enzyme MetH, which functions anaerobically when B12 is present in the growth medium, or the B12-independent enzyme MetE (6). MetK catalyzes the synthesis of SAM from methionine and ATP. The biosynthesis of the branched-chain amino acids isoleucine and valine is also depicted. Gene names are indicated in italics. AHB, α-aceto-α-hydroxybutyrate; DIV, α,β-dihydroxy-isovalerate; DMV, α-β-dihydroxy-β-methylvalerate; KIV, α-ketoisovalerate; KMV, α-keto-β-methylvalerate; RH, ribosylhomocysteine; SAH, S-adenosylhomocysteine. Note that there are three isozymes of acetohydroxy acid synthase (AHAS) in non-K-12 strains of E. coli, encoded by ilvB ilvN (AHAS I), ilvG ilvM (AHAS II), and ilvI ilvH (AHAS III), respectively. In Frag1, which is a K-12 strain of E. coli, AHAS II is nonfunctional because of a frameshift mutation in ilvG (18).