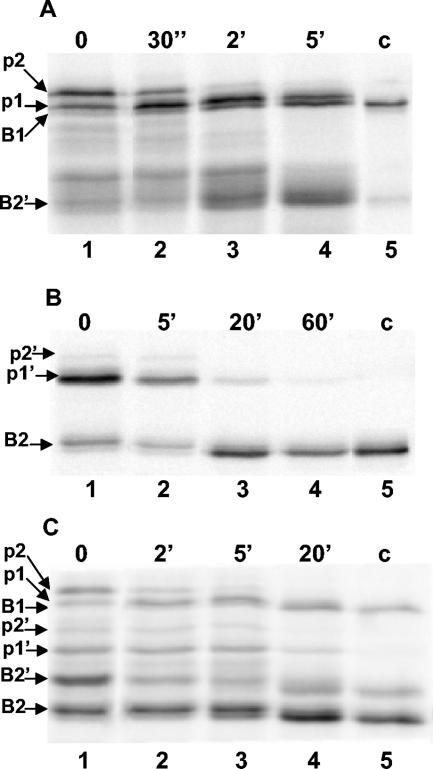
FIG. 2.
Processing of pre-XlnB1 and pre-XlnB2 in S. lividans. S. lividans recombinant strains were grown in a minimal medium containing xylose as carbon source. Mycelia were pulse-labeled for 2 min with [35S]methionine and chased with nonradioactive methionine for the indicated times. Proteins were precipitated with trichloroacetic acid and recovered with the mycelia by centrifugation. After disrupting the mycelia, xylanase-like polypeptides were precipitated with anti-xylanase antibodies, and the products were analyzed by SDS-PAGE and autoradiography. Lanes 1 to 4, xylanase processing; c, control xylanase immunoprecipitated from the culture supernatant after labeling experiment (lane 5); p1 and p2, precursor forms of XlnB1; p1′and p2′, precursor forms of XlnB2; B1, XlnB1; B2, XlnB2; B2′, XlnB2′. A) IAF907-SB1 transformant carrying the plasmid pIAF907-SB1 encoding XlnB1 fused to a Sec-dependent signal sequence. B) IAF907-TB2 transformant carrying the plasmid pIAF907-TB2 encoding XlnB2 fused to a Tat-dependent signal sequence. C) IAF907-SB1TB2 transformant carrying the plasmid pIAF907-SB1TB2 encoding a bicistronic operon with XlnB1 and XlnB2 coding sequences fused, respectively, to a Sec- and Tat-targeting sequence.