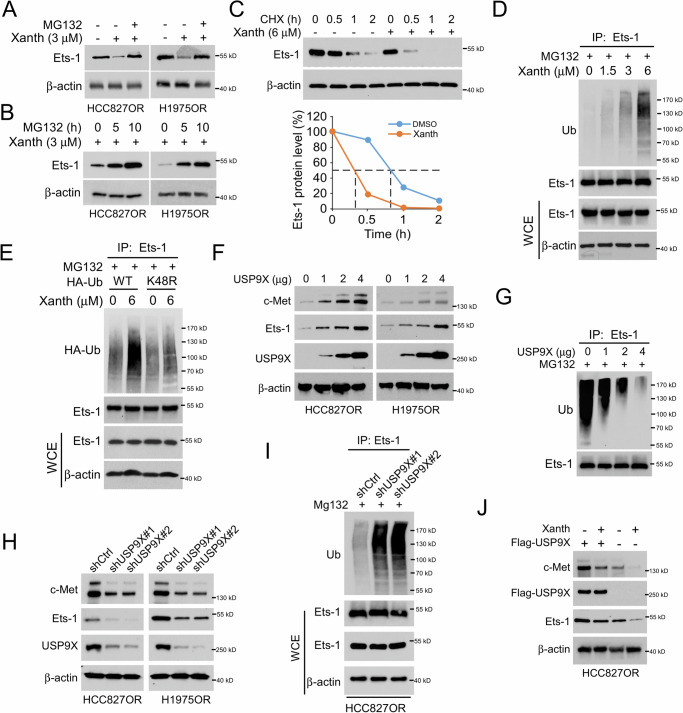
Fig. 5. Xanthohumol promotes ubiquitination and degradation of Ets-1.
A HCC827OR and H1975OR cells were treated with xanthohumol (3 μM) for 24 h, MG132 (10 μM) for 6 h or co-treated, and IB analysis of WCE was performed. B HCC827OR and H1975OR cells were treated with different concentrations of MG132 (0, 5, 10 μM) for 6 h combined with xanthohumol (3 μM) for 24 h, and IB analysis of WCE was performed. C HCC827OR cells were treated with/without xanthohumol (6 μM) for 24 h. Cycloheximide (CHX) was added to the medium and incubated for different times (0, 0.5, 1, 2 h), and IB analysis was performed on WCE. D Treatment with different concentrations of xanthohumol (0, 1.5, 3, 6 μM) for 24 h, followed by incubation with MG132 for 6 h. WCE was collected to detect the ubiquitination level of Ets-1. E After transfection of HA-Ub and K48R plasmids in HCC827OR cells for 24 h, respectively, treatment with or without xanthohumol (6 μM) was given for 24 h, followed by incubation with MG132 for another 6 h. WCE was collected for ubiquitination analysis. F IB analysis of WCE in HCC827OR and H1975OR cells transfected with different masses of USP9X plasmid. G USP9X plasmids of different masses were transfected in HCC827OR cells and after 24 h of transfection, MG132 was added and treated for 6 h. WCE was collected to detect the ubiquitination level of Ets-1. H Stable cell lines with knockdown USP9X were constructed in HCC827OR and H1975OR cells and WCE was collected for IB assay. I In USP9X-deficient HCC827OR cells, MG132 was added and incubated for 6 h, and Ub assay was performed to analyze the ubiquitination level of Ets-1. J In HCC827OR cells, xanthohumol treatment, overexpression of USP9X, or co-treatments were given, and WCE was collected for IB analysis.