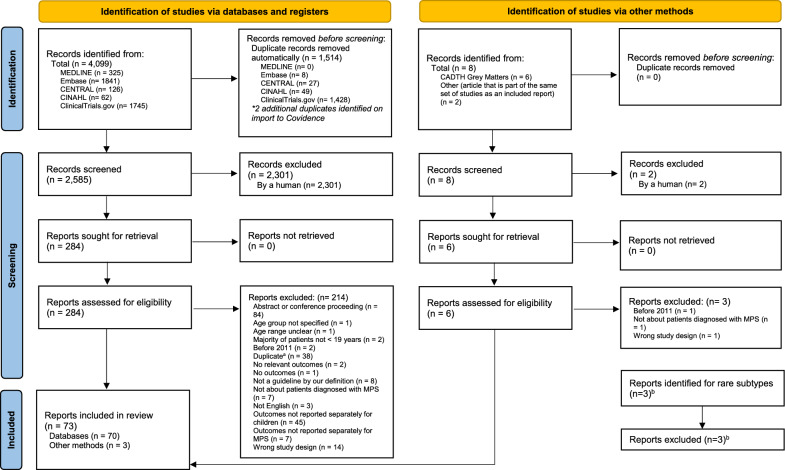
Fig. 2.
PRISMA 2020 flow diagram including searches of databases, registers and other sources. a Duplicates identified in phase 2 were either missed by de-duping tools or were clinical trial registration entries pertaining to published clinical trials included in the review. bDiscussions with clinician scientists regarding rare subtypes not already captured in the review revealed three potentially relevant reports. Since these reports did not meet eligibility criteria and did not include any new potentially relevant outcomes, they were not included in the review. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. https://doi.org/10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/