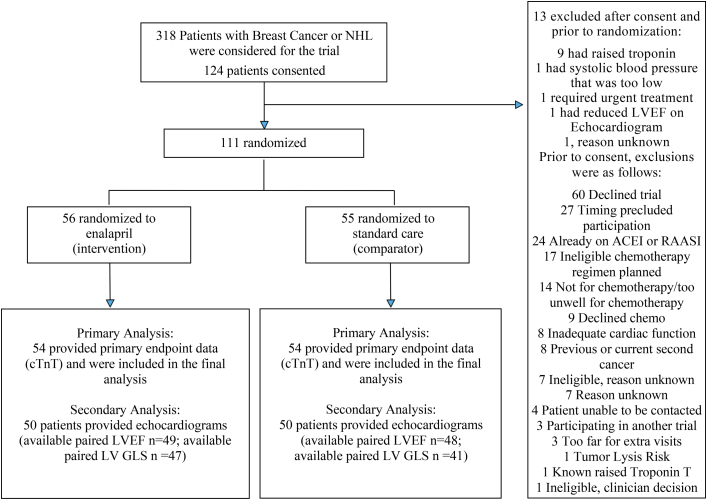
Figure 1.
Participant Flow Throughout the PROACT Trial
This figure presents a CONSORT diagram outlining the flow of participants throughout the PROACT (Preventing Cardiac Damage in Patients Treated for Breast Cancer and Lymphoma) trial. (Top) All patients initially considered for inclusion; (center) the progression to randomization. (Right) Reasons for exclusion both before and after obtaining informed consent. (Bottom) The patients who were included in the analyses for primary and secondary endpoint analyses. ACEI = angiotensin-converting enzyme inhibitor; cTnT = cardiac troponin T; GLS = global longitudinal strain; LV = left ventricular; LVEF = left ventricular ejection fraction; NHL = non-Hodgkin lymphoma; RAAS = renin-angiotensin-aldosterone system inhibitor.