Figure 2.
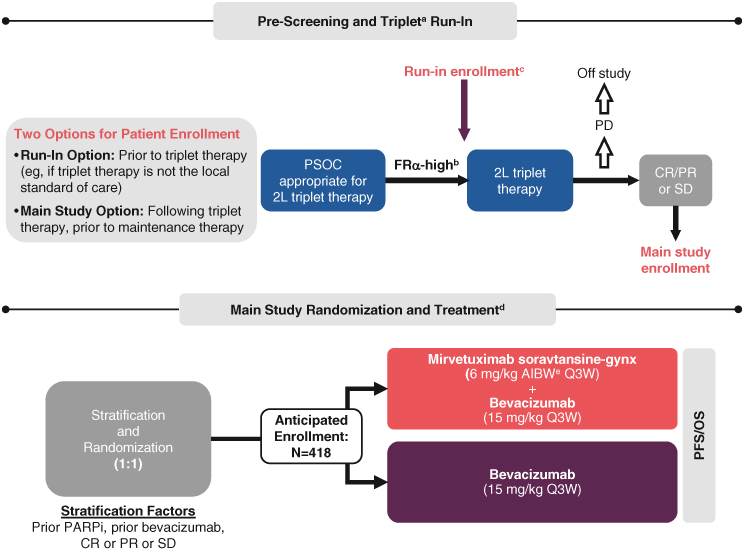
GLORIOSA enrollment and trial design.
aTriplet treatment consists of platinum plus chemotherapy plus bevacizumab for planned six cycles (minimum four and maximum eight cycles), including ≥3 cycles of bevacizumab.
bFRα-high is defined by FRα positivity of ≥75% of tumor membrane staining at ≥2+ intensity (PS2+).
cFRα-high participants who desire to be treated and followed while on their run-in triplet therapy must sign a run-in consent as part of the main consent form if they meet other study eligibility criteria as assessed by the investigator.
dMaintenance treatment must begin ≤12 weeks from last dose of triplet therapy and within 30 days of randomization. Treatment continues until PD, unacceptable toxicity, withdrawal of consent, death or study sponsor termination.
eAIBW, also known as AdjBW, is calculated as IBW (kg) + 0.4 (actual weight – IBW). IBW for females is calculated as 0.9 × height (cm) – 92.
2L: Second-line; AdjBW: Adjusted body weight; AIBW: Adjusted ideal body weight; CR: Complete response; FRα: Folate receptor α; IBW: Ideal body weight; OS: Overall survival; PARPi: Poly (adenosine diphosphate [ADP]-ribose) polymerase inhibitor; PD: Progressive disease; PFS: Progression-free survival; PR: Partial response; PS2+: Positive staining 2+; PSOC: Platinum-sensitive ovarian cancer; Q3W: Three-times per week; SD: Stable disease.
