Abstract
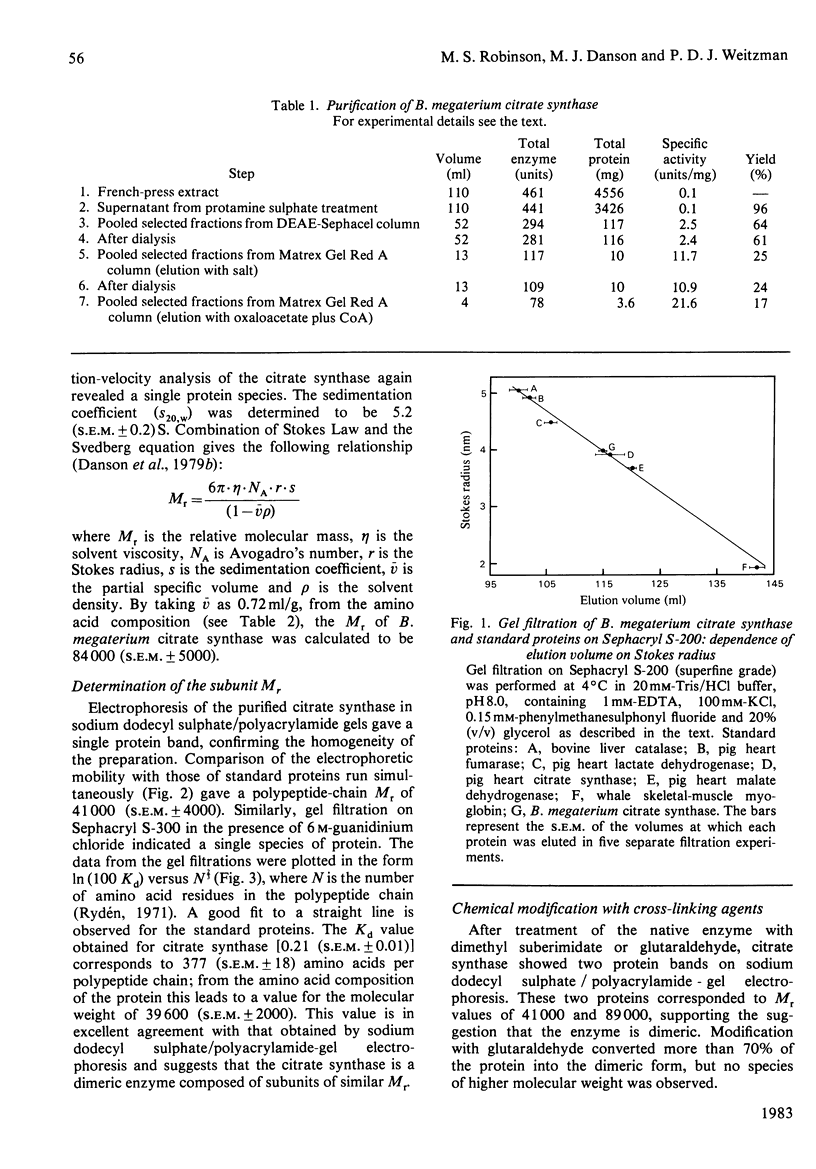
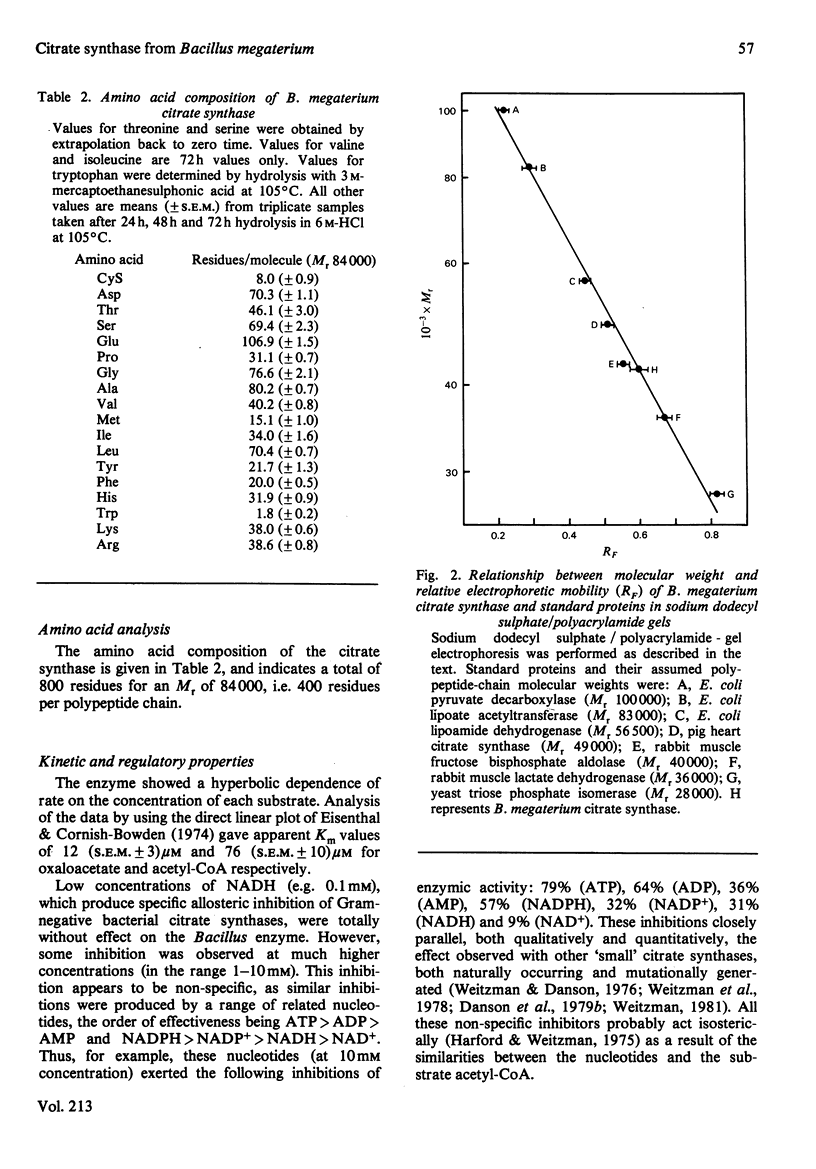
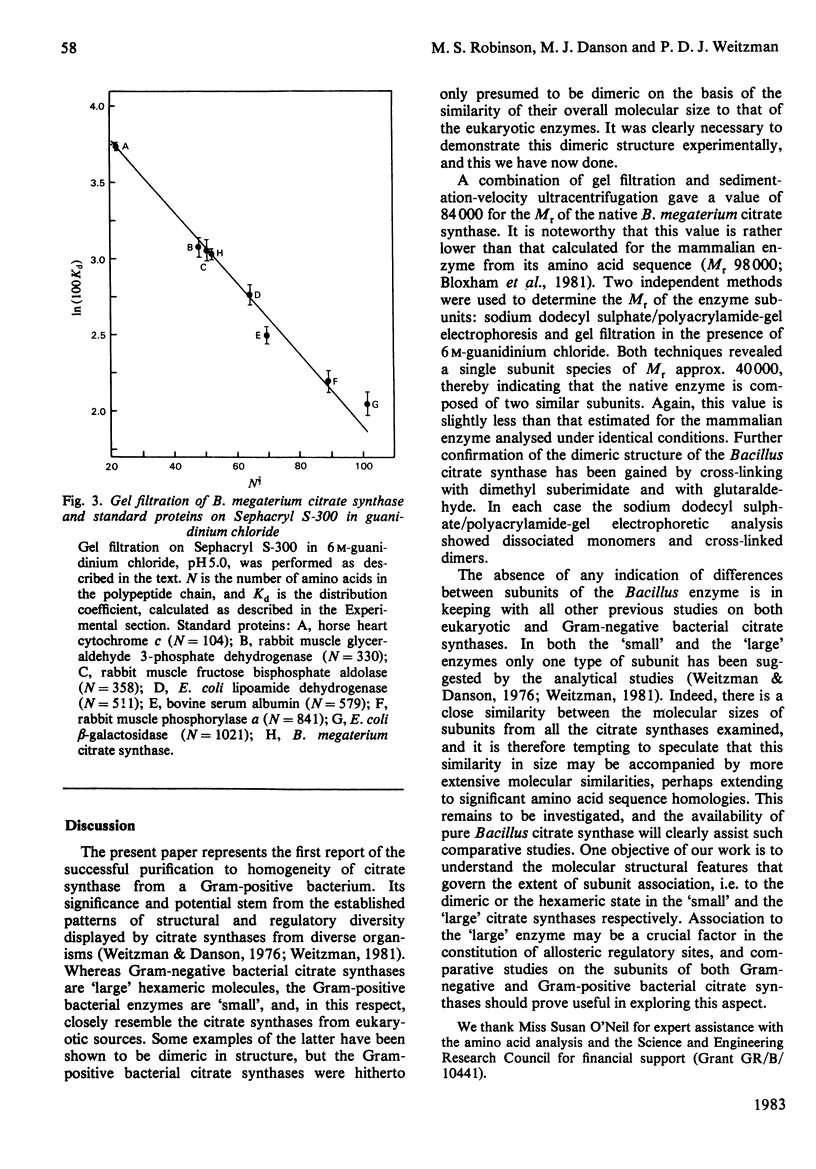
Citrate synthase was purified to homogeneity from a Gram-positive bacterium (Bacillus megaterium) for the first time. The Mr of the native enzyme was determined to be 84 000 (S.E.M. +/- 5000). Sodium dodecyl sulphate/polyacrylamide-gel electrophoresis and gel filtration in guanidinium chloride revealed a single protein species of Mr 40 300 (S.E.M. +/- 4400), indicating a dimeric enzyme. This dimeric structure was confirmed by cross-linking the native enzyme with dimethyl suberimidate and with glutaraldehyde, followed by electrophoretic analysis. The enzyme follows Michaelis-Menten kinetics with respect to both substrates, acetyl-CoA and oxaloacetate, and is sensitive to non-specific inhibition by a range of adenine nucleotides. In both molecular and catalytic properties the citrate synthase closely resembles the enzyme from eukaryotic sources and contrasts markedly with the larger, hexameric, enzyme from Gram-negative bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belew M., Fohlman J. Gel filtration of proteins on Sephacryl S-200 superfine in 6 M guanidine-HCl. FEBS Lett. 1978 Jul 15;91(2):302–304. doi: 10.1016/0014-5793(78)81197-1. [DOI] [PubMed] [Google Scholar]
- Bloxham D. P., Ericsson L. H., Titani K., Walsh K. A., Neurath H. Limited proteolysis of pig heart citrate synthase by subtilisin, chymotrypsin, and trypsin. Biochemistry. 1980 Aug 19;19(17):3979–3985. doi: 10.1021/bi00558a014. [DOI] [PubMed] [Google Scholar]
- Bloxham D. P., Parmelee D. C., Kumar S., Wade R. D., Ericsson L. H., Neurath H., Walsh K. A., Titani K. Primary structure of porcine heart citrate synthase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5381–5385. doi: 10.1073/pnas.78.9.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danson M. J., Hale G., Johnson P., Perham R. N., Smith J., Spragg P. Molecular weight and symmetry of the pyruvate dehydrogenase multienzyme complex of Escherichia coli. J Mol Biol. 1979 Apr 25;129(4):603–617. doi: 10.1016/0022-2836(79)90471-6. [DOI] [PubMed] [Google Scholar]
- Danson M. J., Harford S., Weitzman P. D. Studies on a mutant form of Escherichia coli citrate synthase desensitised to allosteric effectors. Eur J Biochem. 1979 Nov;101(2):515–521. doi: 10.1111/j.1432-1033.1979.tb19746.x. [DOI] [PubMed] [Google Scholar]
- Danson M. J., Porteous C. E. Pyruvate dehydrogenase multienzyme complex of Escherichia coli: determination of the Mr of the lipoate acetyltransferase component. FEBS Lett. 1981 Oct 12;133(1):112–114. doi: 10.1016/0014-5793(81)80483-8. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. The reaction of aldolase with 2-methylmaleic anhydride. Biochem J. 1970 Mar;116(5):843–849. doi: 10.1042/bj1160843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford S., Weitzman P. D. Evidence of isosteric and allosteric nucleotide inhibition of citrate synthease from multiple-inhibition studies. Biochem J. 1975 Nov;151(2):455–458. doi: 10.1042/bj1510455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresze G. B., Dietl B., Ronft H. Mammalian lipoate acetyltransferase: molecular weight determination by gel filtration in the presence of guanidinium chloride. FEBS Lett. 1980 Mar 24;112(1):48–50. doi: 10.1016/0014-5793(80)80124-4. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moriyama T., Srere P. A. Purification of rat heart and rat liver citrate synthases. Physical, kinetic, and immunological studies. J Biol Chem. 1971 May 25;246(10):3217–3223. [PubMed] [Google Scholar]
- Nucci R., Rala C. A., Vaccaro C., Sepe S., Scarano E., Rossi M. Freezing of dCMP aminohydrolase in the activated conformation by glutaraldehyde. J Mol Biol. 1978 Sep 5;124(1):133–145. doi: 10.1016/0022-2836(78)90152-3. [DOI] [PubMed] [Google Scholar]
- Rydén L. Evidence for proteolytic fragments in commercial samples of human ceruloplasmin. FEBS Lett. 1971 Nov 1;18(2):321–325. doi: 10.1016/0014-5793(71)80477-5. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Singh M., Brooks G. C., Srere P. A. Subunit structure and chemical characteristics of pig heart citrate synthase. J Biol Chem. 1970 Sep 25;245(18):4636–4640. [PubMed] [Google Scholar]
- Tong E. K., Duckworth H. W. The quaternary structure of citrate synthase from Escherichia coli K12. Biochemistry. 1975 Jan 28;14(2):235–241. doi: 10.1021/bi00673a007. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D., Danson M. J. Citrate synthase. Curr Top Cell Regul. 1976;10:161–204. doi: 10.1016/b978-0-12-152810-2.50011-5. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D.J., Dunmore P. Regulation of citrate synthase activity by alpha-ketoglutarate. Metabolic and taxonomic significance. FEBS Lett. 1969 Jun;3(4):265–267. doi: 10.1016/0014-5793(69)80154-7. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D., Jones D. Regulation of citrate synthase and microbial taxonomy. Nature. 1968 Jul 20;219(5151):270–272. doi: 10.1038/219270a0. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D., Kinghorn H. A., Beecroft L. J., Harford S. Mutant citrate synthases from Acinetobacter generated by transformation [proceedings]. Biochem Soc Trans. 1978;6(2):436–438. doi: 10.1042/bst0060436. [DOI] [PubMed] [Google Scholar]
- Weitzman P. D. Unity and diversity in some bacterial citric acid-cycle enzymes. Adv Microb Physiol. 1981;22:185–244. doi: 10.1016/s0065-2911(08)60328-8. [DOI] [PubMed] [Google Scholar]
- Wiegand G., Kukla D., Scholze H., Jones T. A., Huber R. Crystal structure analysis of the tetragonal crystal form are preliminary molecular model of pig-heart citrate synthase. Eur J Biochem. 1979 Jan 2;93(1):41–50. doi: 10.1111/j.1432-1033.1979.tb12792.x. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Maeba P., Sanwal B. D. Allosteric regulation of the activity of citrate snythetase of Escherichia coli by alpha-ketoglutarate. Biochem Biophys Res Commun. 1967 Oct 11;29(1):34–38. doi: 10.1016/0006-291x(67)90536-0. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Yang J. T. Physicochemical characterization of citrate synthase and its subunits. J Biol Chem. 1970 Jan 10;245(1):212–218. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]


