Abstract
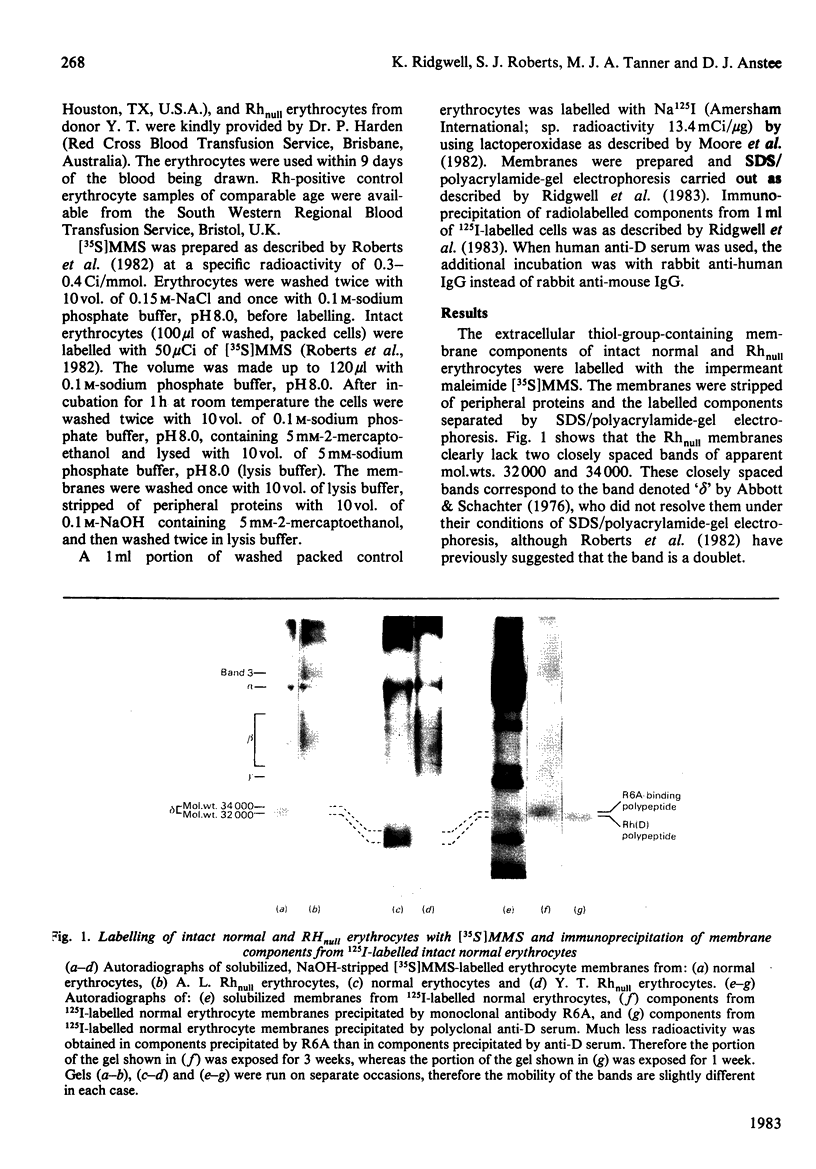
Rhnull human erythrocytes lack all the antigens of the Rhesus blood-group system and are associated with mild chronic haemolytic anaemia. These erythrocytes have an abnormal shape and increased osmotic fragility. Labelling studies with the impermeant maleimide N-maleoylmethionine [35S]sulphone show that Rhnull erythrocytes lack two extracellular thiol-group-containing membrane components of apparent mol.wts. 32 000 and 34 000. Immunoprecipitation with mouse monoclonal antibody R6A (which reacts with all normal erythrocytes, but fails to react with Rhnull erythrocytes) specifically precipitates the 34 000-mol.wt. component from normal erythrocytes. Similar studies with human anti-Rh(D) serum shows that this antibody reacts with the 32 000-mol.wt. component. The results suggest that the R6A-binding polypeptide and the Rh(D) polypeptide may be involved in the maintenance of the shape and viability of the human erythrocyte.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. E., Schachter D. Impermeant maleimides. Oriented probes of erythrocyte membrane proteins. J Biol Chem. 1976 Nov 25;251(22):7176–7183. [PubMed] [Google Scholar]
- Anstee D. J., Edwards P. A. Monoclonal antibodies to human erythrocytes. Eur J Immunol. 1982 Mar;12(3):228–232. doi: 10.1002/eji.1830120311. [DOI] [PubMed] [Google Scholar]
- Edwards P. A. Monoclonal antibodies that bind to the human erythrocyte-membrane glycoproteins glycophorin A and Band 3 [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):334–335. doi: 10.1042/bst0080334. [DOI] [PubMed] [Google Scholar]
- GREEN F. A. STUDIES ON THE RH (D) ANTIGEN. Vox Sang. 1965 Jan-Feb;10:32–53. doi: 10.1111/j.1423-0410.1965.tb04317.x. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G. Molecular identification of the human Rho (D) antigen. FEBS Lett. 1982 Apr 5;140(1):93–97. doi: 10.1016/0014-5793(82)80528-0. [DOI] [PubMed] [Google Scholar]
- Moore S., Woodrow C. F., McClelland D. B. Isolation of membrane components associated with human red cell antigens Rh(D), (c), (E) and Fy. Nature. 1982 Feb 11;295(5849):529–531. doi: 10.1038/295529a0. [DOI] [PubMed] [Google Scholar]
- Ridgwell K., Tanner M. J., Anstee D. J. The Wrb antigen, a receptor for Plasmodium falciparum malaria, is located on a helical region of the major membrane sialoglycoprotein of human red blood cells. Biochem J. 1983 Jan 1;209(1):273–276. doi: 10.1042/bj2090273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S. J., Tanner M. J., Denton R. M. Properties of N-maleoylmethionine sulphone, a novel impermeant maleimide, and its use in the selective labelling of the erythrocyte glucose-transport system. Biochem J. 1982 Jul 1;205(1):139–145. doi: 10.1042/bj2050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgeon P. Hematological observations on the anemia associated with blood type Rhnull. Blood. 1970 Sep;36(3):310–320. [PubMed] [Google Scholar]
- VOS G. H., VOS D., KIRK R. L., SANGER R. A sample of blood with no detectable Rh antigens. Lancet. 1961 Jan 7;1(7167):14–15. doi: 10.1016/s0140-6736(61)92183-3. [DOI] [PubMed] [Google Scholar]



