Abstract
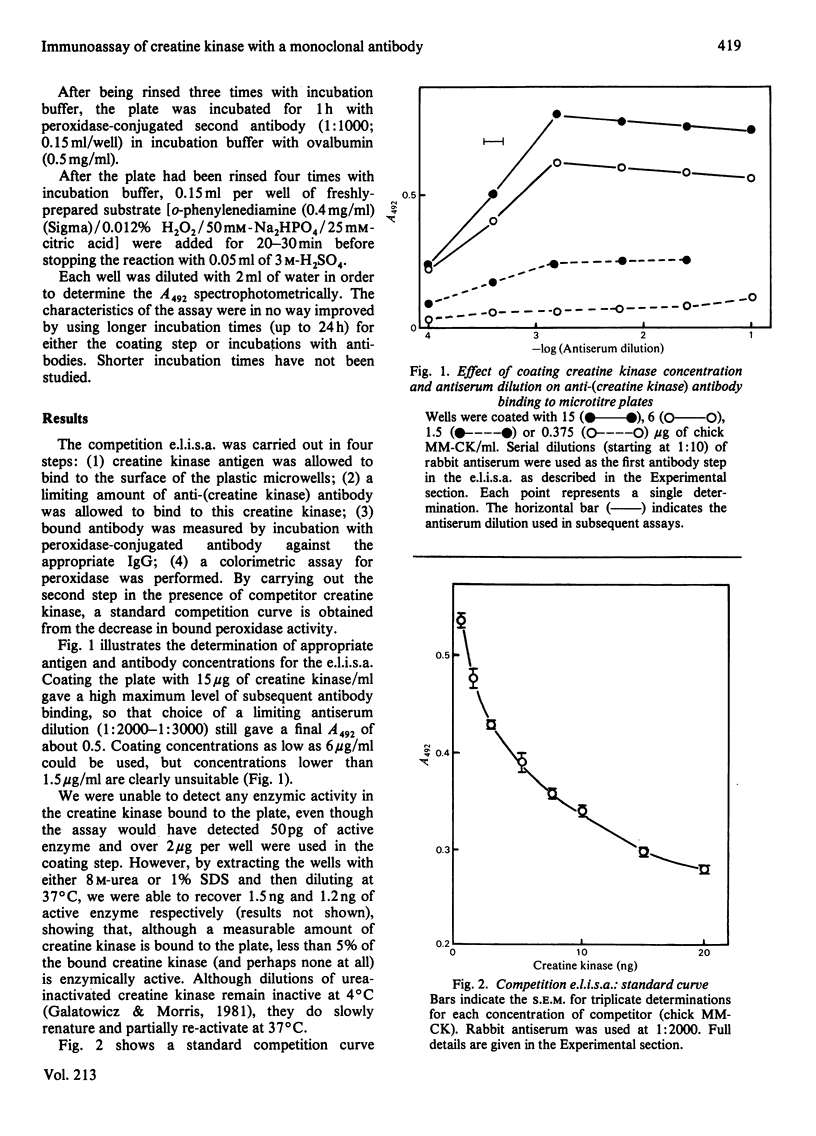
A competition e.l.i.s.a. (enzyme-linked immunosorbent assay) is described that enables direct measurement of the muscle-specific polypeptide of chick creatine kinase (M-CK) in extracts of differentiating muscle-cell cultures and in blood plasma samples, even in the presence of embryonic, or brain-type, creatine kinase. The characteristics of the assay can be considerably improved by the use of a monoclonal antibody, CK-ART, instead of rabbit antisera, and we offer an explanation for this in terms of heterogeneity of antibody affinities in polyclonal antisera. In addition to native enzyme, the assay will measure creatine kinase unfolded and inactivated by 8 M-urea treatment. During chick muscle differentiation in vitro, M-CK increased from 7.5% of the total creatine kinase at 24h to 76.0% at 143h, in good agreement with isoenzyme separation data. As a percentage of the total cell protein, M-CK increased by 156-340-fold over the same period and constituted 0.38-0.56% of the total protein in late cultures. E.l.i.s.a. measurements on 17-20-day embryonic thigh-muscle extracts, which contain almost exclusively M-CK, agree well with enzyme activity and radioimmunoassay. M-CK constituted 0.7-1.6% of the total protein in 17-19-day embryonic thigh muscle. Plasma M-CK concentrations in normal 2-8-week-old chickens were found to be in the range 0.5-0.9 micrograms/ml. Plasma concentrations of 32-56 micrograms/ml were found in 8-week-old dystrophic chickens by both e.l.i.s.a. and enzyme-activity measurements. The results suggest that inactive or unfolded forms of M-CK do not normally exist, in any significant amounts, in cell and tissue extracts or in freshly prepared samples of plasma.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard E. A., Barnard P. J. Use of genetically dystrophic animals in chemotherapy trials and application of serotonin antagonists as antidystrophic drugs. Ann N Y Acad Sci. 1979;317:374–399. [PubMed] [Google Scholar]
- Butler J. E., Feldbush T. L., McGivern P. L., Stewart N. The enzyme-linked immunosorbent assay (ELISA): a measure of antibody concentration or affinity. Immunochemistry. 1978 Feb;15(2):131–136. doi: 10.1016/0161-5890(78)90053-6. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Perriard J. C., Eppenberger H. M. Developmental regulation of creatine kinase isoenzymes in myogenic cell cultures from chicken. Biosynthesis of creatine kinase subunits M and B. J Biol Chem. 1979 Feb 25;254(4):1388–1394. [PubMed] [Google Scholar]
- Cho H. W., Meltzer H. Y. Factors affecting stability of isozymes of creatine phosphokinase. Am J Clin Pathol. 1979 Jan;71(1):75–82. doi: 10.1093/ajcp/71.1.75. [DOI] [PubMed] [Google Scholar]
- Dawkins R. L., Lamont M. Myogenesis in vitro as demonstrated by immunofluorescent staining with antimuscle serum. Exp Cell Res. 1971 Jul;67(1):1–10. doi: 10.1016/0014-4827(71)90614-8. [DOI] [PubMed] [Google Scholar]
- Eppenberger H. M., Dawson D. M., Kaplan N. O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem. 1967 Jan 25;242(2):204–209. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martonosi A., Roufa D., Boland R., Reyes E., Tillack T. W. Development of sarcoplasmic reticulum in cultured chicken muscle. J Biol Chem. 1977 Jan 10;252(1):318–332. [PubMed] [Google Scholar]
- Morin L. G. Creatine kinase: stability, inactivation, reactivation. Clin Chem. 1977;23(4):646–652. [PubMed] [Google Scholar]
- Morris G. E., Cole R. J. Biosynthesis of muscle-specific creatine kinase during differentiation in vitro. FEBS Lett. 1977 Jul 1;79(1):183–187. doi: 10.1016/0014-5793(77)80379-7. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Cole R. J. Calcium and the control of muscle-specific creatine kinase accumulation during skeletal muscle differentiation in vitro. Dev Biol. 1979 Mar;69(1):146–158. doi: 10.1016/0012-1606(79)90281-1. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Cole R. J. Cell fusion and differentiation in cultured chick muscle cells. Exp Cell Res. 1972 Nov;75(1):191–199. doi: 10.1016/0014-4827(72)90536-8. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Head L. P. A monoclonal antibody against the skeletal muscle enzyme, creatine kinase. FEBS Lett. 1982 Aug 16;145(1):163–168. doi: 10.1016/0014-5793(82)81228-3. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Piper M., Cole R. Differential effects of calcium ion concentration on cell fusion, cell division and creatine kinase activity in mucle cell cultures. Exp Cell Res. 1976 Apr;99(1):106–114. doi: 10.1016/0014-4827(76)90685-6. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Piper M., Cole R. Do increases in enzyme activities during muscle differentiation reflect expression of new genes? Nature. 1976 Sep 2;263(5572):76–77. doi: 10.1038/263076a0. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Piper M., Cole R. Quantitative changes in creatine kinase isoenzymes during myogenesis in vitro. Biochem Soc Trans. 1976;4(6):1063–1065. doi: 10.1042/bst0041063. [DOI] [PubMed] [Google Scholar]
- Morris G. E. The use of creatine kinase activity as an index of skeletal-muscle differentiation. Biochem Soc Trans. 1978;6(3):509–511. doi: 10.1042/bst0060509. [DOI] [PubMed] [Google Scholar]
- PEARCE J. M., PENNINGTON R. J., WALTON J. N. SERUM ENZYME STUDIES IN MUSCLE DISEASE. II. SERUM CREATINE KINASE ACTIVITY IN MUSCULAR DYSTROPHY AND IN OTHER MYOPATHIC AND NEUROPATHIC DISORDERS. J Neurol Neurosurg Psychiatry. 1964 Apr;27:96–99. doi: 10.1136/jnnp.27.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARCE J. M., PENNINGTON R. J., WALTON J. N. SERUM ENZYME STUDIES IN MUSCLE DISEASE. III. SERUM CREATINE KINASE ACTIVITY IN RELATIVES OF PATIENTS WITH THE DUCHENNE TYPE OF MUSCULAR DYSTROPHY. J Neurol Neurosurg Psychiatry. 1964 Jun;27:181–185. doi: 10.1136/jnnp.27.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriard J. C., Caravatti M., Perriard E. R., Eppenberger H. M. Quantitation of creatine kinase isoenzyme transition in differentiating chicken embryonic breast muscle and myogenic cell cultures by immunoadsorption. Arch Biochem Biophys. 1978 Nov;191(1):90–100. doi: 10.1016/0003-9861(78)90070-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg U. B., Eppenberger H. M., Perriard J. C. Occurrence of heterogenous forms of the subunits of creatine kinase in various muscle and nonmuscle tissues and their behaviour during myogenesis. Eur J Biochem. 1981 May;116(1):87–92. doi: 10.1111/j.1432-1033.1981.tb05304.x. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- Stewart P. A., Percy M. E., Chang L. S., Thompson M. W. Creatine kinase isozyme transition in chicks with hereditary muscular dystrophy. Muscle Nerve. 1981 Mar-Apr;4(2):165–173. doi: 10.1002/mus.880040214. [DOI] [PubMed] [Google Scholar]
- Tepperman K., Morris G., Essien F., Heywood S. M. A mechanical dissociation method for preparation of muscle cell cultures. J Cell Physiol. 1975 Dec;86(3 Pt 1):561–565. doi: 10.1002/jcp.1040860313. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Gmür R., Lebherz H. G., Siegrist M., Wallimann T., Eppenberger H. M. Differentiation in cultures derived from embryonic chicken muscle. II. Phosphorylase histochemistry and fluorescent antibody staining for creatin kinase and aldolase. Dev Biol. 1976 Feb;48(2):284–307. doi: 10.1016/0012-1606(76)90091-9. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Gmür R., Siegrist M., Burckhardt E., Eppenberger H. M. Differentiation in cultures derived from embryonic chicken muscle. I. Muscle-specific enzyme changes before fusion in EGTA-synchronized cultures. Dev Biol. 1976 Feb;48(2):258–283. doi: 10.1016/0012-1606(76)90090-7. [DOI] [PubMed] [Google Scholar]
- Voller A., Bartlett A., Bidwell D. E. Enzyme immunoassays with special reference to ELISA techniques. J Clin Pathol. 1978 Jun;31(6):507–520. doi: 10.1136/jcp.31.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]


