Abstract
Introduction
The innate branch of the immune system is important in early life, in particular for infants born preterm.
Methods
We performed a longitudinal analysis of the peripheral monocyte compartment in extremely preterm children from a randomized, placebo-controlled study of probiotic supplementation. PBMCs and fecal samples were collected at several timepoints during the first months of life. Monocyte characteristics were analyzed by flow cytometry, and LPS-stimulated PBMC culture supernatants were analyzed by Luminex or ELISA. Plasma cytokines and gut microbiota composition were analyzed by ELISA and 16S rRNA-sequencing, respectively.
Results
The extremely preterm infants had persistent alterations in their monocyte characteristics that were further aggravated in chorioamnionitis cases. They showed a markedly reduced TLR4 expression and hampered LPS-stimulated cytokine responses 14 days after birth. Notably, at later timepoints, TLR4 expression and LPS responses no longer correlated. Sepsis during the first weeks of life strongly associated with increased pro-inflammatory, and reduced IL-10, responses also at postmenstrual week 36. Further, we report a correlation between gut microbiota features and monocyte phenotype and responses, but also that probiotic supplementation associated with distinct monocyte phenotypic characteristics, without significantly influencing their responsiveness.
Conclusion
Extremely preterm infants have monocyte characteristics and functional features that deviate from infants born full-term. Some of these differences persist until they reach an age corresponding to full-term, potentially making them more vulnerable to microbial exposures during the first months of life.
Keywords: Extreme prematurity, Monocytes, Chorioamnionitis, Sepsis, Limosilactobacillus reuteri probiotic supplementation
Introduction
The composition of innate and adaptive immune compartments is dependent on gestational age at birth and markedly differs with regard to phenotypic and functional characteristics between preterm and full-term (FT) neonates [1, 2]. Immunologic immaturities observed in preterm birth are linked to a greater risk of a broad spectrum of health complications, including an increased risk for invasive infections, necrotizing enterocolitis, and sepsis [3]. Extremely preterm infants (born <week 28) are particularly vulnerable [4]. In addition to their overall physiological immaturity, several other factors, like long-time hospitalization, antibiotic treatment, and a delay in enteral feeding, contribute to even more increased susceptibility to infections [4–6].
We recently showed that there are substantial differences between the circulating T- and NK-cell compartments from FT and extremely preterm neonates [7, 8]. As we followed the infants up to a timepoint corresponding to gestational week 36, we could report that there was a somewhat surprisingly rapid immune development within the conventional T-cell compartment [7], also supported by findings in another study [9]. Interestingly, the more innate-like T cells such as γδ T cells but also NK cells were highly affected by extreme prematurity but also by chorioamnionitis before birth and sepsis during the entire study period [8], findings which suggest that cell types linking innate and adaptive branches of the immune system are strongly affected in these infants, even several weeks after birth.
Myeloid cells, including peripheral monocytes, are also known to be affected by prematurity. Several studies have reported alterations regarding phenotype (e.g., surface expression of HLA-DR and toll-like receptors [TLRs]) and some specific functional characteristics (e.g., the production of pro- and anti-inflammatory cytokines upon in vitro stimulation) [10–17]. An impaired monocyte compartment could be of particular importance in premature infants, as monocytes are directly not only involved in the response against infection, but also potent activators of adaptive immune cells. Still, most data on monocyte function in prematurity are generated from cross-sectional studies of preterm infants in general (born up to week 34), while longitudinal studies on monocyte characteristics and functions performed in larger groups of extremely preterm infants are largely lacking.
Surface expression of TLR4 and the production of pro- as well as anti-inflammatory factors following lipopolysaccharide (LPS) stimulation are common markers of general monocyte responsiveness. In preterm infants, TLR4 expression and LPS responsiveness vary between studies, and results are mainly from cord-blood-derived monocytes, reviewed in [12]. When studying peripheral blood monocytes following LPS stimulation during the first week of life in preterm infants (<week 32), their TLR4 expression was similar to that of healthy FT controls, while the production of reactive oxygen intermediates was enhanced [17].
Sepsis, a common complication in preterm infants, could be a result of poor innate immune responses to microbes [18]. Still, monocyte frequencies are not necessarily influenced by sepsis [19] and infants with late-onset sepsis mount a strong in vivo innate response, characterized by pro- and anti-inflammatory cytokines in plasma [20].
It is well established that the gut microbiota is important for immune development in early life [21], including monocyte function and regulation [22]. Further, neonatal monocytes are reported to have impaired extravasation into tissues, which could in turn impact gut homeostasis and immune responsiveness [23]. For the healthy term infant, the fundamental change from the microbe-deprived in utero environment is usually without complications [24]. In the case of preterm neonates, colonization might be more problematic, as the mucosal tissues in the intestine are immature and more prone to inflammation and infection. Further, changes in microbial composition, as well as a reduced diversity, are reported in very preterm and extremely preterm infants [25]. We, and others, have reported on a reduced abundance of Bifidobacteria and Bacteroides, accompanied by an increased abundance of Enterobacteriaceae and Staphylococcus [26–28].
Probiotic supplementation of preterm infants has shown to counteract dysbiosis and reduce morbidities, but also to reveal strain-specific differences [29, 30]. Microbiota maturation in extremely preterm neonates can be predicted by hospitalization length as well as gestational age at birth, but can also be increased by probiotic supplementation [31]. We recently showed that probiotic supplementation with Limosilactobacillus (L.) reuteri DSM 17938 to extremely preterm neonates resulted in successful colonization of the probiotic product despite antibiotic usage. Supplemented neonates had a higher bacterial diversity, as well as lower relative abundance of Proteobacteria at phylum level, and Staphylococcaceae and Enterobacteriaceae at family level during the first week of life [28, 32].
To address the current knowledge gap regarding monocyte development and maturation after birth in extremely preterm infants, we performed a comprehensive, longitudinal analysis of peripheral monocytes during the first months of life of extremely preterm children participating in a randomized, placebo-controlled study of probiotic supplementation [33]. We analyzed the ability of the monocytes to respond to microbial stimulation and interact with T cells, together with a comprehensive analysis of pro- and anti-inflammatory-secreted mediators following in vitro microbial stimulation. The obtained immune data were further analyzed in relation to pregnancy complications, the development of sepsis, gut microbiota features as well as probiotic supplementation. We demonstrate that extreme prematurity clearly links to persistent alterations in monocyte characteristics over the study period including the ability of monocytes to interact with T cells, which was most prominent in the group where chorioamnionitis was reported. Further, extremely preterm neonates have a robust LPS responsiveness at later timepoints after birth, in spite of a markedly reduced TLR4 expression on monocytes. Sepsis during the first weeks of life strongly associated with an increased pro-inflammatory and reduced IL-10 response at the time when the infants had reached term-equivalent age. Further, we report a correlation between gut microbiota features and monocyte phenotype and responses, but also that probiotic supplementation associates with monocyte phenotypic characteristics without significantly influencing their responsiveness.
Materials and Methods
Study Design and Sampling Timepoints
Prophylactic Probiotics to Extremely Low Birth Weight Preterm Infants (PROPEL) is a prospective, double-blinded, randomized-controlled, multicenter cohort study where extremely preterm, low birth weight infants were supplemented with the probiotic L. reuteri DSM 17938 or placebo. The study design has been described in detail elsewhere [33] (ClinicalTrials.gov. [NCT01603368]) and was approved by the Ethics Committee for Human Research in Linköping, Sweden (Dnr 2012/28‐31, Dnr 2012/433‐32). In brief, neonates born between gestational week 23+0 and 27+6, with a birthweight less than 1,000 g, were eligible for enrolment within 3 days after delivery. Clinical perinatal and neonatal data on the preterm infants, including information on clinical chorioamnionitis, preeclampsia, and neonatal sepsis, were entered prospectively in a case report form, and the parameters relevant for this study are shown in online supplementary Table S1 (for all online suppl. material, see https://doi.org/10.1159/000541468). Peripheral blood was collected at day (D) 14, D28, and at a timepoint corresponding to postmenstrual week (W) 36+0, as well as from healthy FT infants (born between W 38 and 42) without any neonatal complications, at 14 days of age. Fecal samples were collected from the preterm group at D7, D14, D21, D28, and W36. The sampling schemes for blood and feces and number of neonates sampled at each timepoint are summarized in online supplementary Table S2a, b, respectively. In all subsequent figures, the following abbreviations are used: FT D14 (samples from FT infants at day 14 after birth), extremely low birthweight (ELBW) D14; ELBW D28 and ELBW W36 (samples from ELBW infants at day 14, day 28, and W 36+0, respectively).
Probiotic Supplementation
The probiotic supplementation protocol has been delineated in detail elsewhere [33]. Briefly, supplementation of L. reuteri DSM 17938 (1.25 × 108 bacteria/day) or placebo started within 3 days of age and was given on daily basis until postmenstrual week (W) 36+0 (online suppl. Table S1). The study product and placebo were provided by BioGaia AB (Stockholm, Sweden) in identical oil suspensions. Quality of the product was regularly checked by the manufacturer, and the concentration of L. reuteri was within the stipulated limits in all batches used in the trial, as described in [33].
Diagnosis of Sepsis
The diagnosis of sepsis included the combination of positive blood and/or cerebrospinal fluid culture, clinical deterioration, and laboratory-confirmed inflammatory response. The bacteria detected in positive cultures included Gram-negative rods such as Escherichia coli and Klebsiella, and Gram-positive Streptococcus group B, Enterococcus, Staphylococcus aureus, and coagulase-negative staphylococci. Characteristics of the sepsis cases are depicted in online supplementary Table S1 where both culture-proven sepsis and clinical sepsis cases were included. For culture-proven sepsis, a diagnosis required a positive blood and/or cerebrospinal fluid culture together with a minimum of 2 out of 4 of the following: (1) white blood cell count ≤5 or ≥20 × 109 cells/L; (2) total platelet count ≤100 × 109 cells/L; (3) CRP ≥15 mg/L; and (4) newly recognized apnea, elevated oxygen demand, or requirement of respiratory support. In case of a positive culture of coagulase-negative staphylococci, the culture-proven sepsis diagnosis required two separate positive blood cultures with the same antibiotic resistance pattern and/or a central venous/arterial line prior to sepsis onset together with at least one of the laboratory criteria (1–3) mentioned before and clinical deterioration (criterion 4). For clinical sepsis, a diagnosis required a negative blood culture and at least three out of the mentioned criteria (1–4) for culture-proven sepsis.
Isolation of Peripheral Blood Mononuclear Cells and Plasma Separation
Peripheral blood was drawn by venous or arterial puncture. The blood volume varied substantially between the neonates and timepoints. At D14 and D28, 0.8 mL blood was collected in custom-made 1.5-mL Eppendorf tubes with heparin as anticoagulant. The heparin was of injection quality and bought from the local hospital pharmacy. At W36, 2.5 mL blood was collected in 4-mL Vacuette tubes (Hettich, Sweden). The heparin tubes containing the blood samples were centrifuged for 10 min at 200 g. Plasma was removed into cryotubes and frozen at −80°C. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) gradient separation. Briefly, the blood was diluted in prewarmed RPMI-1640 (GE Healthcare Life Sciences, Hyclone Laboratories, UT) and then layered on the top of the Ficoll (Ficoll:blood ratio 3:5), followed by centrifugation at 400 g without brake for 30 min at room temperature. The white cloudy interface, containing PBMCs, was collected. Isolated PBMCs were washed three times in warm RPMI-1640 containing 2% FCS for 15 min at 400 g with low brake, and cell viability was determined using trypan blue exclusion assay. PBMCs were then resuspended in freezing medium containing 40% RPMI-1640, 50% fetal calf serum (Sigma-Aldrich, St. Louis, MO), and 10% DMSO (Sigma-Aldrich) at a concentration of 5 × 106 cells/mL. Cells were gradually frozen in a freezing container (Mr. Frosty) at 80°C freezer, and after 24 h, cells were transferred to the liquid nitrogen until further analysis.
Flow Cytometry
Frozen PBMCs were thawed gently at 37°C in the water bath, washed 3 times in RPMI-1640 supplemented with 20 mm HEPES (GE Healthcare Life Sciences), counted using trypan blue exclusion assay, and seeded into round-bottomed 96-well tissue culture plate (Costar, Corning Incorporated, ME) at a concentration of 0.25 × 106 cells/well. The cells were acclimated for 2 h at 37°C with 5% CO2 before the preparation for staining. Cells were then transferred to V-shaped staining plates and stained with the LIVE/DEAD Fixable Dead Cell Stain Kit-Aqua (Life Technologies, Eugene, OR) according to the manufacturer’s instruction. Blocking of cell surface Fc receptor was done with 10% human serum. Staining of cell surface markers was performed using the following antibodies from BioLegend (San Diego, CA): CD14 (clone: HCD14), CD80 (clone: 2D10), CD86 (clone: IT2.2), HLA-DR (clone: L243), TLR4 (clone: HTA125), and CD11b (clone: ICRF44). All antibodies were directly conjugated to the fluorochromes as shown in online supplementary Table S3a. Stained cells were washed, resuspended in FACS wash buffer, and acquired using FACS Verse instrument using FACS Suite software (BD Biosciences). Reference settings were created in order to generate the compensation matrix which is calculated on single-color fluorescence to correct for the spectral overlap or spillover values. The samples were then run on that compensation setting. The FACS panel and gating strategies for FACS analysis are shown in online supplementary Table S3a and Figure S1, respectively. Fluorescence-minus-one and isotype controls were used for gating.
In vitro Stimulation
Frozen PBMCs were thawed and washed three times with RPMI-1640. Cells were counted using a hemocytometer (Marienfeld, Germany), and viability was determined with trypan blue staining. Subsequently, the cells were resuspended in cell culture medium at a concentration of 2.5 × 106/mL, consisting of RPMI-1640 supplemented with 20 mm HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mm L-glutamate (2 mm) (all GE Healthcare Life Sciences), and 10% heat-inactivated FCS (Sigma-Aldrich). For stimulations, 200 μL of the PBMC suspension (5 × 105 cells) was added per well, in a flat-bottomed 96-well tissue culture plate, stimulated for 24 h with or without 1 μg/mL LPS (Sigma-Aldrich), and incubated at 37°C and 5% CO2 (Costar, Cambridge, UK). After 24-h incubation, supernatants were collected and kept at −80°C until analyzed with Luminex as described below.
Multiplex Analysis and ELISA
Cell culture supernatants were centrifuged at 350 g for 10 min to get rid of any cell debris present in the supernatants. The levels of cytokines and chemokines in the supernatants from the PBMC stimulations were analyzed using Millipore’s MILLIPLEX magnetic bead-based detection kits (EMD Millipore Corporation, Billerica, MA, USA), assay performed according to the manufacturer’s instructions. The MILLIPLEX Panel 1 (8-plex), MILLIPLEX Panel 2 (30-plex), MILLIPLEX Panel 3 (14-plex), and MILLIPLEX Panel 4 (6-plex) were used for analysis of a total of 25 analytes (online suppl. Table S3b). For panel 4, the samples were diluted 1:100 in RPMI media with 10% FCS. To the plate, 25 μL of standard or control and culture supernatant from each respective infant was added to appropriate wells, according to the manufacturer’s instructions. Samples were run in single wells and not duplicated. Plates were analyzed using a Luminex 200 analyzer with MasterPlex CT control software and MasterPlex QT analysis software (MiraiBio, San Bruno, CA, USA). Standard curves for each analyte were generated using standards provided by manufacturer. Sandwich ELISA was performed for the detection of IL-23 (Invitrogen, Waltham, MA, USA), as duplicate samples from the culture supernatant according to the manufacturer’s instruction. Plasma LL-37 (Hycult Biotech, Wayne, PA, USA) and cytokines TNF, IL-6, sCD14, and sCD163 (R&D Duo Set) were measured using sandwich ELISA according to the instructions from the manufacturer.
16S rRNA Gene Sequencing
The microbial profiling based on the V3–V4 hypervariable region of the 16S rRNA gene was performed from fecal samples as previously described [34]. A synthetic mock microbial community was also prepared alongside the samples and used to determine potential contaminants. An amplicon sequence variant (ASV) table was generated from demultiplexed fastq files as previously described [28]. ASVs identified as Archaea (2 ASVs), Eukaryota (119 ASVs), cyanobacteria (5 ASVs), chloroplasts (1 ASV), as well as ASVs that were not identified at phylum (53 ASVs) level, were filtered out. The synthetic mock community prepared alongside the samples was used to remove potential contaminants as described [34]. Additionally, the order Rhizobiales was also removed (25 ASVs). Rhizobiales are nitrogen fixation soil bacteria and have been shown to be contaminants in the neonatal intensive care unit environment [35].
Microbiota-Immune Marker Associations
The fecal samples were collected at D7, D14, D21, D28, and W36, while the blood samples were collected at D14, D28, and W36. To investigate associations between monocyte characteristics or function with the microbiota within the preterm group, samples were paired as follows: fecal D7 and D14 with blood D14, fecal D7 to D28 with blood D28, and fecal D7 to W36 with blood W36, resulting in an average of 29 samples paired per group (range between 13 and 41; online suppl. Table S2b). The immune data selected for the study were the data from the flow cytometry experiments (geometric mean fluorescence intensity combined with the cellular frequencies) and the LPS:unstimulated ratios of cytokines and chemokines analyzed with Luminex from the in vitro stimulations.
Statistical Analyses
Statistics for Immunological Data
GraphPad Prism 7 (GraphPad Software, La Jolla, CA) was used for the statistical analyses. Graphs were displayed either as box and whiskers showing min to max, or as symbol and lines. The results were shown as medians with interquartile ranges for continuous variables with skewed distributions shown in the figures. Mann-Whitney U test was used to assess differences between groups. The nonparametric Kruskal-Wallis test followed by the Dunn’s multiple comparisons test was performed to compare age-related differences within the ELBW group of preterm infants. The Spearman correlation test was used to analyze correlation between variables. Pearson’s χ2 test was used for categorical outcome variables. Fisher’s exact test was used when the observed frequency for any cell was less than five. Results were considered significant when p < 0.05, and actual p values were displayed in each figure. The principal component analyses (PCAs) were performed in GraphPad Prism V10.2.3. Different combinations of cell subset frequencies and expression levels were reduced to two principal components. The variance in the data explained by one component is mentioned as a percentage on the axis. The total variance in the data that is explained by the two components combined is mentioned in the graphs. The validity of the PCA was checked with the Kaiser-Meyer-Olkin measure of sampling adequacy and the Bartlett’s test of sphericity.
Statistics for Associations between Bacterial Communities and Immune Markers
Prior to statistical analyses, the immune data were log-transformed. Alpha-diversity was calculated using Shannon’s diversity index, Pielou’s evenness index, and richness assessed as number of observed ASVs, using the diverse package [36]. Associations between α-diversity and immune data were explored using Pearson correlation (|r| >0.4, p < 0.05; Hmisc package). Relationships between centered log-ratio transformed genus and family-level taxa, and the immune data were explored using a partial least squares regression analysis (using the spls function in the mixOmics package, which maximizes the covariance between the two data sets) [37]. The results were depicted using a relevance network graph with a cutoff > |0.5| and visualized in a heatmap. The statistical analyses were performed in R version 4.0.3 (R Core Team 2020).
Results
Monocytes from Extremely Preterm Infants Have an Altered Capacity to Interact with T Cells and Respond to LPS throughout the Postnatal Period up to a Timepoint Equivalent to FT
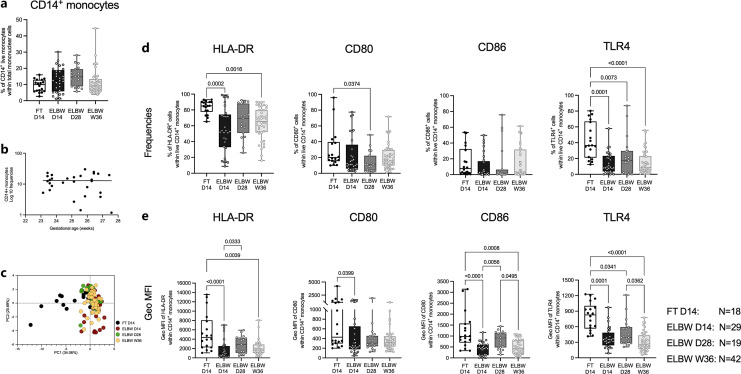
PBMCs collected at D14, D28, and W36 from extremely preterm infants and at D14 from FT infants were examined for the relative proportions of total live CD14+ monocytes and their expression of CD11b, HLA-DR, CD80, CD86, and TLR4 (online suppl. Fig. S1). The frequency of total CD14+ monocytes did not show significant differences between preterm and FT neonates at D14. Monocyte percentages were relatively stable during the study period in the preterm neonates (mean frequencies ±SD; FT D14 9.27 ± 3.8; ELBW D14 12.98 ± 7.6; ELBW D28 14.78 ± 6.2; ELBW W36 11.5 ± 7.7) and did not seem to be influenced by gestational age at birth (Fig. 1a, b). A subsequent analysis of monocyte phenotypic characteristics revealed notable changes between the FT and preterm infants that persisted during the study period (Fig. 1c). Extended analysis revealed that, in particular, the percentages of HLA-DR and TLR4-expressing CD14+ monocytes were markedly reduced in preterm neonates at D14 compared to FT neonates, and these frequencies also remained low at D28 and W36 (Fig. 1d). Also, the expression levels of HLA-DR, CD86, and TLR4 were significantly reduced on monocytes from the preterm group during the entire study period (Fig. 1e). CD11b expression did not differ between preterm and FT infants (online suppl. Fig. S2). Within the preterm group, the proportion of CD11b-expressing monocytes dropped at W36, compared to D28, but did not differ compared to FT infants. Subdividing the monocytes into CD11b+ and CD11b− populations revealed that the abovementioned differences were attributed to the CD11b+ monocyte subset, although there were similar trends in the CD11b− subset (online suppl. Fig. S2).
Fig. 1.
HLA-DR and TLR4 expression on peripheral monocytes is impaired by extreme prematurity. Peripheral blood samples from 18 FT infants (day 14 after birth) and 90 ELBW preterm infants (day 14, day 28 after birth, and postmenstrual week 36+0) were collected, and phenotypic characterization of peripheral monocyte was performed by multicolor flow cytometry. a Comparison of the frequencies of total live CD14+ monocytes between FT D14 and ELBW preterm infants at different postnatal timepoints. b Correlation between the percentages of monocytes and the gestational age in ELBW D14 neonates. c PCA comparing the monocyte phenotype (both frequencies and expression levels of HLA-DR, CD80, CD86, and TLR4 on total CD14+ as well as CD11b+/− cells within CD14+ monocytes). Frequencies (d) and expression levels (e) of monocyte-associated HLA-DR, CD80, CD86, and TLR4 of FT and longitudinal ELBW preterm infants. Box and whisker plots show median as the central line, and error bars represent minimum to maximum values. Kruskal-Wallis test with Dunn’s multiple comparison was used for group comparison. Spearman correlation test was used to analyze correlation between gestational age and the proportions of monocytes from ELBW D14 infants.
Frequencies of CD86+ monocytes, but no other studied monocyte features, were inversely correlated with the gestational age at birth (online suppl. Fig. S3a). There were no differences in monocyte characteristics at any timepoint that could be attributed to infant sex, twin pregnancy, or mode of delivery (online suppl. Fig. S3b–d).
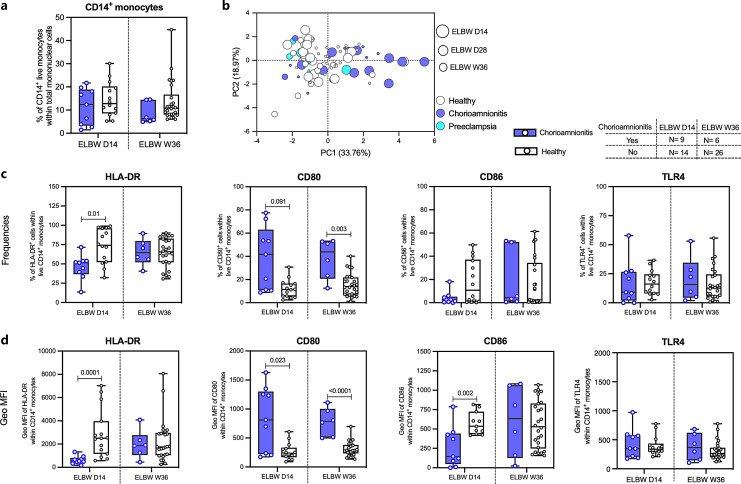
Chorioamnionitis Associates with the Functional Phenotype of CD14+ Monocytes from Extremely Preterm Infants
We next investigated whether two common pregnancy complications, clinical chorioamnionitis (or intra-amniotic infection) and/or preeclampsia, related to infant monocyte characteristics. Clinical chorioamnionitis did not associate with the monocyte frequencies among the extremely preterm infants (Fig. 2a), but clearly correlated with the monocyte phenotype, which was not seen for preeclampsia cases (Fig. 2b). Investigating the markers individually, we observed that the frequency of HLA-DR+ CD14+ monocytes and expression level of HLA-DR were reduced at D14, but not at W36, in the chorioamnionitis group (Fig. 2c, d). In contrast, the frequency of CD80+ CD14+ monocytes and expression level of CD80 were increased in the chorioamnionitis group at both D14 and W36 (Fig. 2c, d). For CD86, the results were less clear with no differences in percentages, but a lower expression level at D14 in the chorioamnionitis group (Fig. 2c, d). TLR4 expression was not altered in chorioamnionitis cases (Fig. 2c, d). Analyses of these markers at D28 were not possible due to a low number of samples in the chorioamnionitis group at this timepoint.
Fig. 2.
Chorioamnionitis associates with altered HLA-DR and CD80 expression on monocytes. a Comparison of monocyte frequencies between healthy and chorioamnionitis-exposed preterm neonates from D14 and W36. b PCA of the monocyte compartment including frequencies and expression levels of HLA-DR, CD80, CD86, and TLR4 on total CD14+ cells from healthy and chorioamnionitis- and preeclampsia-exposed preterm neonates. Proportions (c) and expression levels (d) of monocyte-associated HLA-DR, CD80, CD86, and TLR4 of healthy and ELBW (D14 and W36) preterm infants encountered with chorioamnionitis. Interleaved box and whisker plots show median as the central line, and error bars represent minimum to maximum values. The Mann-Whitney U test was used for comparison between healthy and chorioamnionitis-exposed group.
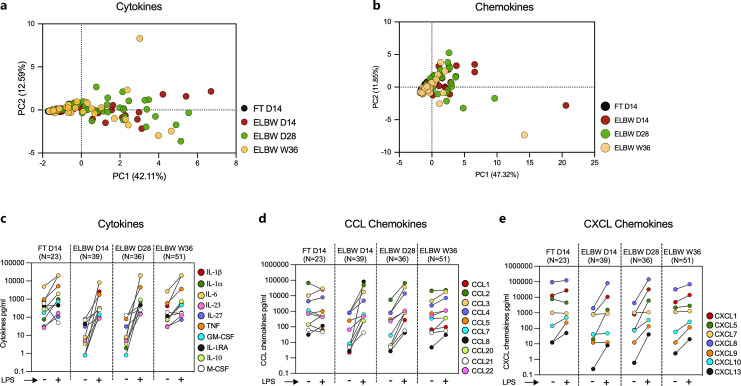
Robust Monocyte Cytokine and Chemokine Responses following LPS Stimulation of Cells from Extremely Preterm Infants
As TLR4 expression was markedly reduced on the CD14+ monocytes in extremely preterm infants, we continued to study how their PBMCs responded to LPS in terms of cytokine and chemokine production. A total of 27 cytokines and chemokines were analyzed by multiplex assay (n = 26) (online suppl. Table S3b) or ELISA (n = 1). When normalized to the unstimulated secretion, a PCA revealed some differences between preterm and FT infants, as well as differences within the preterm group related to postnatal age, in particular for the cytokines (Fig. 3a, b for cytokines and chemokines, respectively). An elaborated analysis revealed that these differences were primarily attributed to unstimulated values, as the spontaneous production of most pro- and anti-inflammatory cytokines and chemokines was markedly low or negligible in the extremely preterm infants compared with FT infants, in particular at D14 and D28 (Fig. 3c–e for an overview, online suppl. Fig. S4a–c; Tables S4, S5 for details and statistics). At D14, extremely preterm infants had a lower LPS response in terms of all cytokines except for M-CSF, including the commonly investigated IL-1α, IL-6, TNF, and IL-10. This low responsiveness was restored at the later timepoints and then equaled that observed from cells from FT infants at D14 (Fig. 3c; online suppl. Fig. S4d; Table S4). Further, LPS stimulation triggered a robust secretion of most of the measured chemokines at all timepoints. With the exception of CXCL9, CXCL10, and CXCL13, chemokine responses in extremely preterm infants equaled or exceeded (CCL1, CCL2) those of FT infants already at D14 (Fig. 3d, e; online suppl. Fig. S4e, f; Table S4). There were no significant differences in cytokine or chemokine production that could be related to infant sex, twin pregnancy, mode of delivery, or pregnancy complications such as preeclampsia and chorioamnionitis (online suppl. Fig. S5).
Fig. 3.
LPS exposure elicits robust secretion of monocyte-associated cytokines and chemokines in ELBW preterm infants. PBMCs from D14 FT and D14, D28, and W36 preterm infants were stimulated with LPS or left unstimulated for 24 h. A total of 27 monocyte-associated cytokines and chemokines from the cell culture supernatant were measured in different Luminex panels and by ELISA. PCA comparing the ratios of LPS-stimulated and LPS-unstimulated values of different cytokines (a) and chemokines (b) in FT and ELBW preterm children at different postnatal ages. Extended analyses (c–e) compared the unstimulated (LPS−) versus LPS-stimulated (LPS+) values where each point (depicted with distinct color) with matched data (LPS− and LPS+) spread across the row represented median value of each cytokine (c) or chemokine (d, e) from infants of different age groups. Elaborated comparisons showing individual data for each cytokine and chemokine were presented in online supplementary Fig. S4.
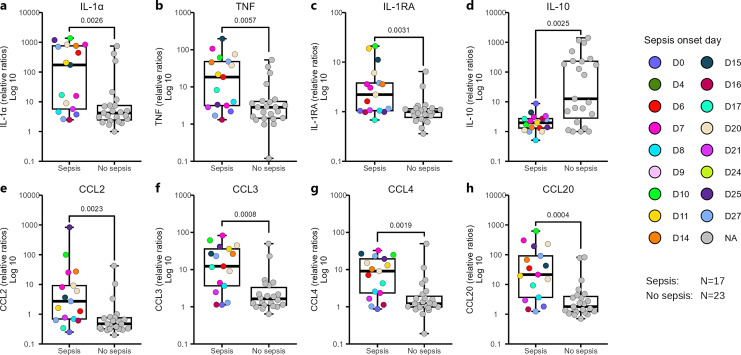
Sepsis Onset during the First Four Weeks of Life Associates with Persistently Skewed LPS Responses, toward an Enhanced Pro-Inflammatory Profile
Due to low numbers of culture-proven sepsis cases in the group of extremely preterm infants where cells were available for phenotypic analysis, we were not able to monitor monocyte characteristics in relation to sepsis. However, among the cell culture supernatants from LPS-stimulated PBMCs from W36 (from Fig. 3) there were sufficient numbers of culture-proven sepsis cases to analyze cytokine responses in relation to sepsis. Information on the individuals included in Figure 4 is displayed in online supplementary Table S1. There were augmented levels of the pro-inflammatory cytokines IL-1α and TNF in infants with a previous sepsis episode (Fig. 4a, b). For the anti-inflammatory cytokine IL-RA, levels were generally very low but still higher in the culture-proven sepsis group (Fig. 4c), while secretion of IL-10 was markedly low in the neonates with a previous sepsis episode (Fig. 4d). Several of the studied chemokines with pro-inflammatory characteristics were also elevated in extremely preterm infants with a previous culture-proven sepsis compared to the group with no previous sepsis (Fig. 4e–h for CCL2, CCL3, CCL4, and CCL20, respectively).
Fig. 4.
Sepsis impacts the magnitude of cytokine and chemokine production in response to LPS in W36 ELBW preterm infants. a–h showing cytokine and chemokine responses following LPS exposure at W36 for individuals with previous sepsis episodes* in comparison with individuals where no sepsis was reported. Data in the graph were presented as log 10 values of the ratios of LPS-stimulated and LPS-unstimulated groups. Box and whisker plots show median as the central line, and error bars represent minimum to maximum. Colors represented the day of sepsis onset. The Mann-Whitney U test was used for group comparison. *Only culture-positive sepsis cases were included in the analysis, and the clinical sepsis cases were excluded.
Sepsis May Influence the Inflammatory Profile in Plasma
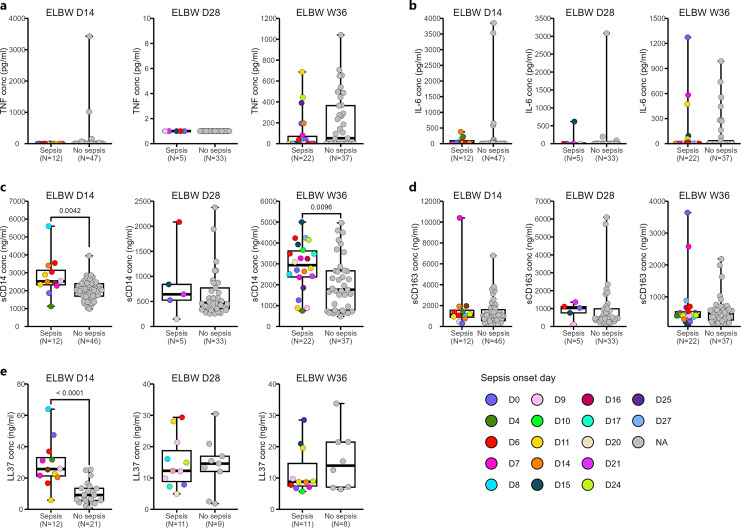
Next, we wanted to investigate whether the sepsis-associated inflammatory profile of monocytes stimulated in vitro shown above could be detected also in plasma. Accordingly, we measured TNF, IL-6, sCD14, and sCD163 – all markers associated with inflammatory responses and/or monocyte activation. We also determined the level of LL-37, a human cathelicidin frequently associated with infection and sepsis. Overall, these factors had a diverse longitudinal profile (online suppl. Fig. S6). Plasma levels of TNF were very low and hardly detectable at the early timepoints, while IL-6 was detected at both early and late timepoints, with a clear drop at D28 (online suppl. Fig. S6a, b). In contrast, sCD14 and sCD163 were both readily detected in plasma from the preterm neonates. While sCD14 levels showed a marked and strong drop at D28 compared to the other two timepoints (online suppl. Fig. S6c), sCD163 gradually decreased during the study period (online suppl. Fig. S6d). LL-37 did not seem to be influenced by age (online suppl. Fig. S6e).
While plasma levels of TNF and IL-6 were unrelated to previous sepsis episodes (Fig. 5a, b), sCD14 was enhanced at D14 and W36 in cases where sepsis onset had occurred before the sampling timepoint (Fig. 5c). For sCD163 however, there were no differences related to previous sepsis at any timepoint (Fig. 5d). LL-37 levels increased at D14 in the group of neonates with a sepsis onset before D14 (Fig. 5e).
Fig. 5.
The systemic inflammatory profile is altered by sepsis. Plasma samples from D14, D28, and W36 from the extremely preterm children were analyzed for the detection of TNF, IL-6, sCD14, sCD163, and antimicrobial peptide LL-37 by ELISA. Box and whisker plots visualized the plasma levels of TNF (a), IL-6 (b), sCD14 (c), sCD163 (d), and LL-37 (e) in infants having or not having sepsis at different postnatal ages. Each point with distinct color depicted the onset day of sepsis for each infant. The Mann-Whitney U test was used for group comparisons.
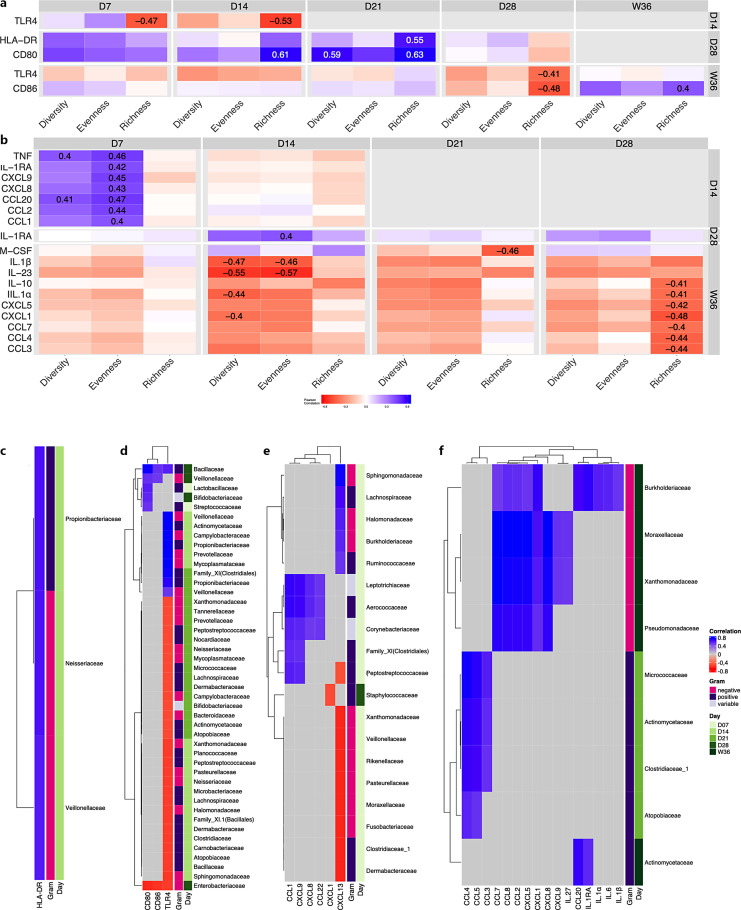
Gut Microbiota Traits Associate with Monocyte Phenotypic and Functional Characteristics in Extremely Preterm Infants
In the extremely preterm infants, microbial richness was negatively associated with the frequency and expression levels of TLR4, while it was mainly positively associated with CD80, CD86, and HLA-DR expression (Fig. 6a). Specifically, we found that higher microbial richness during the first 2 weeks of life (D7 and D14) as well as D28 was associated with lower TLR4 expression at D14 and W36, respectively. On the contrary, higher microbial richness at D14 and D21 was positively associated with expression of CD80 and HLA-DR at 1 month of age (D28). At D28, richness and CD86 were negatively associated, but the pattern was the opposite at W36. For monocyte cytokine and chemokine responses, the LPS:unstimulated ratio either negatively associated with or did not correlate to α-diversity measures, with the exception of D7, where microbiota diversity and evenness positively correlated with several cytokines and chemokines, including TNF and CCL20 (Fig. 6b).
Fig. 6.
Gut microbiota traits associate with monocyte phenotypic and functional characteristics in extreme prematurity. a, b Heatmap showing Pearson correlation of fecal microbiota α-diversity measures and immune markers of ELBW preterm infants. Diversity: Shannon index, evenness: Pielou’s evenness index, richness: observed ASVs. The significant differences (|r| >0.4, p < 0.05) are depicted by displaying the correlation coefficient. c–f Heatmaps showing pair-wise similarities between taxa at family-level and the immune markers, obtained from the latent components of the partial least squares (PLS) regression analysis after a cutoff >|0.5|. c Flow cytometry data at D14. d Flow cytometry data at D28. e Luminex data at D28. f Luminex data at W36.
To further investigate the association between the fecal microbiota and the immune markers, a partial least squares regression analysis was performed. The majority of the associations between the phenotypic flow cytometry data and the fecal microbiota were found when comparing flow cytometry data from D28 and microbiota analysis at D7–28 (Fig. 6c, d). Most associations were observed for TLR4 expression and the fecal microbiota composition, where TLR4 expression at D28 mainly showed a negative correlation with several bacterial families at D14 and D21. Moreover, at D28, the expression of CD80 and CD86 as well as TLR4 clearly negatively associated with Enterobacteriaceae but positively with Bacillaceae (Fig. 6d). For the secreted cytokines and chemokines following LPS stimulation, there were mainly positive associations between the LPS:unstimulated ratio and the fecal microbiota (Fig. 6e, f), with the exceptions of CXCL13 that was negatively associated with several taxa at D7, and CXCL1 that was negatively associated with Staphylococcaceae at D28 (Fig. 6c).
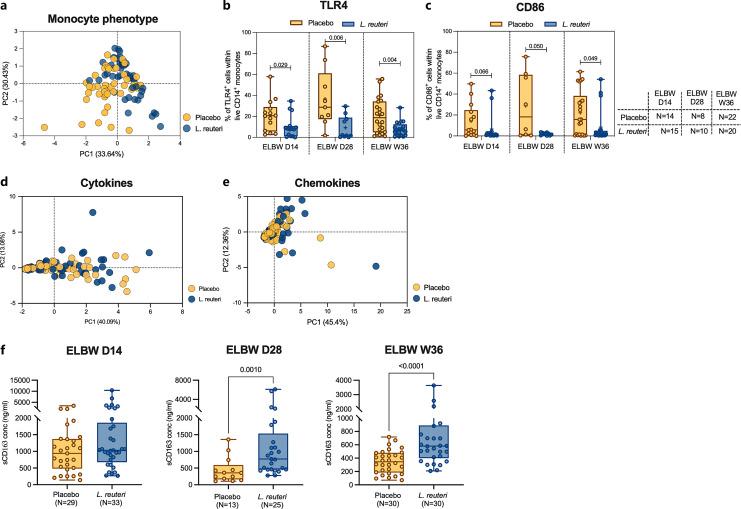
L. reuteri Supplementation Strongly Associates with Monocyte Phenotypic Characteristics but Not with Monocyte Responses
Throughout the study, we noted a substantial variation between the extremely preterm babies regarding both monocyte phenotypic and functional characteristics. Therefore, we further analyzed whether these parameters differed between those infants that were supplemented with L. reuteri and the placebo controls (online suppl. Table S1). Notably, L. reuteri supplementation significantly correlated with overall monocyte characteristics (Fig. 7a), differences attributed to reduced frequencies of monocytes expressing both TLR4 and CD86 in the L. reuteri-treated group (Fig. 7b, c). Still, there were no differences in LPS responsiveness between supplemented and placebo groups in terms of differences in cytokine or chemokine responses (Fig. 7d, e; online suppl. Fig. 7) following LPS stimulation in vitro. Finally, when investigating if plasma levels of monocyte-associated factors were linked to probiotic supplementation, we also observed elevated levels of sCD163 at D28 and W36 (Fig. 7f).
Fig. 7.
L. reuteri supplementation affects monocyte phenotypic characteristics but has marginal effects on functional responses. a PCA plot of monocyte phenotype comparing placebo and L. reuteri-treated group of ELBW preterm infant at different postnatal timepoints. Explained variances of each principal component (PC) were indicated in brackets. Interleaved box and whisker plots showed proportions of TLR4 (b) and CD86 (c) expressing monocytes comparing placebo and L. reuteri-treated group at different postnatal ages. PCA of LPS induced cytokine (d) and chemokine (e) response comparing between placebo and L. reuteri-treated group in ELBW preterm children at all timepoints. f Box and whisker plots envisaged soluble CD163 levels in plasma from placebo and L. reuteri-treated groups at D14, D28, and W36 ELBW preterm neonates. The Mann-Whitney U test was used to make group comparisons.
Discussion
Considering the enhanced risk of infections in extremely preterm neonates, the functional characteristics of the innate branch of the immune system are of high relevance for their ability to survive during the first months of life. To the best of our knowledge, we are the first to report how phenotypic and extensive functional characteristics in monocytes develop over time in extremely preterm infants (all born before week 28, weighing less than 1 kg; many born as early as 23–24 weeks of gestation) starting from D14 after birth and continuing to a timepoint corresponding to FT. Further, we also had the possibility to link immune readouts to clinical characteristics during the study period, as well as to gut microbiota composition and probiotic supplementation.
We were able to demonstrate that while extreme prematurity did not associate with peripheral monocyte numbers or monocyte subpopulations, it largely related to the functional phenotype, i.e., the expression of receptors important for microbial signaling (TLR4), as well as for activation of and interaction with T cells (HLA-DR, CD80/86). These phenotypic differences were persistent up to term-equivalent age, affected LPS-stimulated responses at D14, but not during later timepoints. Chorioamnionitis significantly correlated with monocyte phenotypic characteristics, while sepsis clearly associated with higher pro-inflammatory secretory profile and lower IL-10 after stimulation, but also with increased levels of factors reflecting monocyte activation in plasma. Monocyte characteristics could be connected to the gut microbiota profile, some of which were also linked to probiotic supplementation.
The frequency of monocytes was not altered in extremely preterm infants at any timepoint investigated, nor did it relate to gestational age at birth. However, HLA-DR expression on monocytes was largely reduced during the entire study period, suggesting that monocyte–T-cell collaboration is hampered for a significant amount of time after birth in extremely preterm infants. Our data on reduced HLA-DR expression are in agreement with previous reports on very preterm infants (born <33 weeks of gestation), showing low frequencies of HLA-DR+ monocytes up to 7 days after birth [13, 16], although we did not observe any correlation between HLA-DR expression and gestational age at birth in our study that was seen before [13]. Clinical chorioamnionitis, but not preeclampsia, was strongly associated with a lower HLA-DR expression at day 14, but this did not last until week 36. In agreement with other findings in very preterm neonates and at earlier timepoints after birth [10], this could suggest that chorioamnionitis negatively influences the crosstalk between monocytes and T cells the first weeks after birth.
Monocytes express both CD80 and CD86. Both serve as ligands for CD28 and CTLA4, stimulating and regulatory receptors on T cells, respectively. Although often mentioned in the same context, CD80 and CD86 have low sequence homology as well as different characteristics and affinity for their ligands; there are now an increasing number of studies that implicate separate functions of the two receptors [38–40]. While CD86 seems to be more constitutively expressed on human myeloid cells in vitro, CD80 is upregulated upon stimulation [40]. Notably, we observed that in cases with clinical chorioamnionitis, the expression of CD80 increased significantly in terms of both the frequency of CD80-positive cells and expression levels at D14 and W36, while CD86 appeared to have a somewhat reverse pattern at D14. This is interesting as placental dysfunction has been reported to influence monocyte gene expression in cord blood from preterm infants. In pregnancies with placental inflammation, neonatal monocytes showed an enhanced transcriptional expression of genes related to monocyte activation [41], and our data with an increased CD80/CD86 ratio in chorioamnionitis cases are in line with this reasoning. An increase in CD80 and a decrease in CD86 have also been reported in adult sepsis patients [42], further supporting a differential role of these receptors during infection. Together with the results on the reduced HLA-DR above, these findings could suggest that maternal chorioamnionitis could trigger an inflammatory profile of the preterm newborn infant that is unspecific and does not lead to useful interactions with T cells (co-stimulation without proper antigen trigger).
Further, the ability to respond to Gram-negative bacteria, reflected by TLR4 expression, was also significantly reduced in monocytes from extremely preterm infants. This is in contrast to data generated from cord blood samples of moderately preterm and FT infants, where TLR4 expression was similar between the two groups [17, 43]. Further, opposite to a previous longitudinal study on TLR expression in preterm infants born up to week 36 [14], we did not see a recovery of TLR4 expression in our cohort of extremely preterm infants. Instead, it remained significantly reduced compared to FT infants throughout the entire study period. TLR4 is readily expressed in human placenta [44], and preterm birth and infection are linked to alterations in expression as well as TLR4-mediated signaling in the intrauterine environment in many studies [45, 46]. Although we did not evaluate the intrauterine environment per se in our study, we observed that TLR4 expression on infant peripheral monocytes was not influenced by chorioamnionitis. This could suggest that the monocytes of the preterm infant are more affected by the actual prematurity rather than intrauterine infections, in contrast to the markers related to monocyte interactions with T cells (HL-DR and CD80/CD86) that were also affected by chorioamnionitis.
CD11b is an integrin receptor highly expressed on several cells from the myeloid lineage including circulating monocytes. Its role on human monocytes is not fully elucidated, but it is associated with both pro- and anti-inflammatory properties [47]. However, it is implicated in both myeloid cell interaction with T cells and in the regulation of TLR4 responses. Further, as it is implicated in monocyte homeostatic extravasation after birth [23], it is also of interest to evaluate in preterm infants. A previous study showed an elevated baseline expression of CD11b in preterm compared with FT infants, although CD11b-positive and CD11b-negative subpopulations did not differ in their LPS responsiveness [17]. When subdividing the monocytes into CD11b+ and CD11b−, it was clear that the CD11b+ monocytes dominated in both FT and preterm infants, and that the HLA-DR reduction seen within the preterm group was mainly attributed to the CD11b-expressing subpopulation. Regarding the reduced TLR4 expression reported in the preterm group, it was evident in both CD11b+ and CD11b− monocytes, warranting further studies on the role of CD11b in TLR4 signaling.
Upon LPS challenge, we could observe a lower production of LPS-triggered cytokines, e.g., IL-1α, IL-6, TNF, and IL-10 at D14 in cells from the extreme preterm infants, in line with previous work [15]. The data suggest that the low production of pro-inflammatory cytokines at D14 relates to the low TLR4 expression on the monocytes from the extreme preterm infants. Another study examining the LPS response of preterm neonates during the first week of life observed elevated TLR4 levels after LPS exposure in vitro in a preterm group of infants compared to FT controls, which was also linked to high production of reactive oxygen intermediates, showing that premature monocytes robustly respond in a pro-inflammatory manner to microbial stimulation [17]. The longitudinal perspective is important, as we clearly show that the extreme preterm infants had recovered their LPS responsiveness already at D28, and they continued to show a robust LPS responsiveness also at W36 in spite of the sustained reduced TLR4 expression. This suggests that there is no linear correlation between TLR4 expression and LPS responsiveness, further supported by the resilient production of chemokines following LPS stimulation throughout the study period. In addition, although pregnancy complications have been suggested to result in subsequent LPS hyporesponsiveness [10], neither chorioamnionitis nor preeclampsia were linked to LPS responses at any timepoint, showing the importance of a longitudinal study design with a long-term follow-up period as well as the importance of studying both phenotypic and broad functional readouts when evaluating immune responses in very young children. LPS responsiveness could also have been influenced by cellular composition of the PBMC samples, which in turn could relate to gestational age at birth. Monocyte frequencies have been shown to increase with gestational age in preterm infants [2], although we could not see this in our cohort, and they were further stable during the study period.
In our work, we did not investigate repeated exposures to microbial substances. Monocyte training after an initial BCG exposure has been reported in adult monocytes, but is much less pronounced in neonatal monocytes, where a more tolerogenic response is observed [48]. It is not known how preterm monocytes respond to immune training and how it influences their subsequent responses, but as this is likely to influence future immune fitness, further studies in the field are warranted.
Unfortunately, among the preterm samples available for phenotypic analyses, there were too few sepsis cases to evaluate its role for the expression of monocyte surface characteristics, but TLR4 expression has previously been implicated as a biomarker for neonatal late-onset sepsis [49]. Increased CD11b expression has also been described in extreme preterm infants developing sepsis [50]. In the group of extremely preterm infants, it was evident that the frequency of CD11b+ cells was high at D14 and D28, but significantly lower at W36. If this reflects a longer time since a previous sepsis episode at W36 than at D14 and D28 is not clear. However, the CD11b+ cell frequencies in the preterm group at D14 did not differ from those in FT infants at D14, indicating that the lower percentages at W36 might reflect a general maturation feature.
Extrapolating the production of cytokines and chemokines following LPS stimulation to previous episodes of sepsis, we observed that the pro-inflammatory factors IL-1α and TNF, the anti-inflammatory IL-1RA but also the chemokines CCL2, CCL3, CCL4, and CCL20 measured in W36 samples were significantly higher after sepsis. In contrast, there was a markedly lower production of IL-10. This suggests that sepsis will imprint the monocyte population mainly in a pro-inflammatory way that can be detected several weeks or even months later. Although it is difficult to directly prove in humans, data from murine models of sepsis support the importance of myeloid cells for survival and recovery [3]. This sepsis imprinting was further mirrored in the plasma compartment, as previous sepsis associated with higher sCD14 levels, but also higher levels of the antimicrobial peptide LL-37. Elevated production of LL-37 most likely mirrors an active antimicrobial response, perhaps also from epithelial cells which are the main LL-37 producers. Soluble CD14 is a nonspecific marker of monocyte activation, but it can also make nonimmune cells like epithelial cells LPS responsive [51]. Further, the levels of sCD14 in plasma have been connected to severity and prognosis in several infectious diseases including sepsis in neonates [52]. Notably, factors usually associated with inflammation and also with sepsis, like IL-6 and TNF, were detected in plasma samples from very few individuals and then at exceptionally low levels. This could question the biological relevance of these factors in plasma in this context, but it should also be remembered that the sampling timepoints in this study might not always have matched the time frame where these factors reached their peak levels in circulation.
Gut microbiota traits are suggested to impact immunity, in particular in early life. Although molecular mechanistic studies on how this immune imprinting occurs are difficult to perform in humans, there is a growing body of data to support that certain gut microbiota traits can protect the neonate from infections and sepsis [53–55]. An increased α-diversity, e.g., richness, during the first weeks of life associated with lower TLR4 expression but higher HLA-DR and CD80 expression in the extremely preterm infants. Further analysis revealed that TLR4 expression negatively correlated with many bacterial families including Enterobacteriaceae but positively correlated with Bacillaceae. Regarding cytokine and chemokine responses following LPS stimulation, they generally negatively associated with α-diversity measures, except for D7. Further, there were mainly positive associations between LPS-stimulated chemokines in particular and the fecal microbiota. Although our microbiota results are likely to be confounded by antibiotic usage, the negative associations between different microbiota features and TLR4 expression are worth noticing. It is possible that the dysbiosis repeatedly observed in prematurity [25–28] negatively influences monocyte features. However, a low TLR4 expression this early in life could also be protective and a way to limit LPS hyperresponsiveness that has been reported for neonatal monocytes [56], in order to avoid an exaggerated pro-inflammatory response triggered by the maturing microbiota with increased diversity.
In addition, we noted that the group of preterm infants receiving L. reuteri supplementation had lower TLR4 expression than the placebo controls. This could support the idea of a protective immune profile induced by the probiotic supplementation, making it less likely to overreact during the early microbiota maturation period. As functional responses at D28 and W36 in extreme preterm infants were similar to those of FT babies, despite low TLR4 expression, this could further suggest that low TLR4 expression is not linked to a compromised immune system. Also, the supplemented group of infants had markedly elevated levels of sCD163 in plasma compared to the placebo controls. On the CD14+ monocyte surface, the scavenger receptor CD163 is highly expressed and strongly upregulated by IL-10 [57–59], and it is often associated with anti-inflammatory responses, immune suppression but also tumorigenic responses [60]. The cleaved form – sCD163 – is frequently reported as a plasma biomarker in different diseases, frequently parallels with TNF responses, and is often associated with poor outcomes. However, it is also connected to various regulatory responses, including anti-inflammatory activities and clearing components released upon red blood cell lysis [61, 62]. It is possible that its role as a scavenger clearing the circulation of toxic hemoglobin breakdown products is beneficial in extreme prematurity [62]. How the probiotic supplementation could exert immune regulation in vivo is not known. However, we have shown that the L. reuteri strain used in this particular study has strong monocyte-dependent regulatory features in vitro [63]. We have also found that this strain induces an atypical and more tolerogenic memory-like phenotype in human mucosa-like moDCs [64] and that it promotes barrier integrity in intestinal epithelial cells [65].
We acknowledge some limitations of our study, mainly due to limitations in cell and plasma availability as well as combined data on microbiota composition, as described in both the Result and Discussion sections. Accordingly, not all individuals were included for all analyses, although the number of individuals included in longitudinal characterizations was high. The limits in sample availability prevented extensive surface marker characterization and plasma cytokine profiling. Also, all experiments are performed on samples that have been frozen, and the handling procedures might have altered cell characteristics, behavior, and cytokine concentrations compared to unprocessed fresh samples. Further, cell vulnerability, in particular for D14 samples, where a lower overall PBMC viability (primarily observed within the lymphocyte population) [7, 8] could be observed for some donors, could have impacted the results. Finally, we did not have possibility to stimulate with multiple microbial ligands for different periods.
In the same study cohort, we recently showed how unconventional T cells, as well as NK cells, were influenced by extremely preterm birth during the entire study period [8]. Together with our findings reported here on the monocyte compartment, we conclude that the innate arm of the immune system is strongly affected in extremely premature infants also at term-equivalent age, further complicated by causes of pre- and postnatal inflammation like chorioamnionitis and neonatal sepsis, respectively. Still, and somewhat surprising, innate immune responses to LPS were robust already at D28, despite a markedly lower TLR4 expression than FT infants. This is likely to be of major importance for the extremely preterm neonate who is highly reliant on innate immunity to manage the establishment of the commensal microbiome and immune development, processes complicated by the exposure to antibiotics and immunosuppressive corticosteroids in the ICU environment.
Acknowledgments
We thank all the participants and their parents for their cooperation and the nurses involved in blood drawings.
Statement of Ethics
The study design has been described in detail elsewhere [28] and registered at ClinicalTrials.gov (NCT01603368) and was approved by the Ethics Committee for Human Research in Linköping, Sweden (Dnr 2012/28‐31, Dnr 2012/433‐32). Written informed consent was obtained from parents within 3 days after delivery.
Conflict of Interest Statement
Eva Sverremark-Ekström has received honoraria for lectures and a grant for another research project from BioGaia AB. Thomas Abrahamsson has received honoraria for lectures and a grant for the present trial from BioGaia AB. Maria C Jenmalm has also received honoraria for lectures from BioGaia AB. The other authors have no conflict of interest to declare.
Funding Sources
This research was funded by the Swedish Research Council (Dnr 2016-01715, 2020-01839 and 2023-02616 to E.S.-E.; and 921.2014-7060 to T.A.), BioGaia AB (T.A.), the Cancer and Allergy Foundation to E.S.-E., the Hesselman Foundation to E.S.-E., the Golden Jubilee Memorial Foundation to E.S.-E., the Freemasons of Sweden and Stockholm University to E.S.-E., the Swedish Society for Medical Research to T.A., the Swedish Society of Medicine to T.A., the Research Council for South-East Sweden to T.A., ALF Grants to T.A., Region Östergötland to T.A., Medical Inflammation and Infection Center (MIIC), and Linköping University to T.A.
Author Contributions
Study design: E.S.E., T.A., and M.J.; funding acquisition: E.S.E. and T.A.; patient inclusion and sample collection: T.A.; experimental design: K.R.Q., T.A., M.J., and E.S.E.; experimental work: K.R.Q., D.G., and M.M.; data analyses: K.R.Q., D.G., M.M., Y.d.J., and G.B.J.; data interpretation and critical review of the manuscript: all coauthors. Writing of the manuscript: K.R.Q., M.M., and E.S.E.
Funding Statement
This research was funded by the Swedish Research Council (Dnr 2016-01715, 2020-01839 and 2023-02616 to E.S.-E.; and 921.2014-7060 to T.A.), BioGaia AB (T.A.), the Cancer and Allergy Foundation to E.S.-E., the Hesselman Foundation to E.S.-E., the Golden Jubilee Memorial Foundation to E.S.-E., the Freemasons of Sweden and Stockholm University to E.S.-E., the Swedish Society for Medical Research to T.A., the Swedish Society of Medicine to T.A., the Research Council for South-East Sweden to T.A., ALF Grants to T.A., Region Östergötland to T.A., Medical Inflammation and Infection Center (MIIC), and Linköping University to T.A.
Data Availability Statement
All data generated and analyzed during the study period are included in the manuscript and the online supplementary materials. Further queries can be directed to the corresponding author.
Supplementary Material.
Supplementary Material.
References
- 1. Anderson J, Thang CM, Thanh LQ, Dai VTT, Phan VT, Nhu BTH, et al. Immune profiling of cord blood from preterm and term infants reveals distinct differences in pro-inflammatory responses. Front Immunol. 2021;12:777927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peterson LS, Hedou J, Ganio EA, Stelzer IA, Feyaerts D, Harbert E, et al. Single-cell analysis of the neonatal immune system across the gestational age continuum. Front Immunol. 2021;12:714090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kan B, Razzaghian HR, Lavoie PM. An immunological perspective on neonatal sepsis. Trends Mol Med. 2016;22(4):290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Norman M, Hallberg B, Abrahamsson T, Björklund LJ, Domellöf M, Farooqi A, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;321(12):1188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fleiss N, Tarun S, Polin RA. Infection prevention for extremely low birth weight infants in the NICU. Semin Fetal Neonatal Med. 2022;27(3):101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawn JE, Ohuma EO, Bradley E, Idueta LS, Hazel E, Okwaraji YB, et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet. 2023;401(10389):1707–19. [DOI] [PubMed] [Google Scholar]
- 7. Qazi KR, Bach Jensen G, van der Heiden M, Björkander S, Holmlund U, Haileselassie Y, et al. Extremely preterm infants have significant alterations in their conventional T cell compartment during the first weeks of life. J Immunol. 2020;204(1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rahman Qazi K, Jensen GB, van der Heiden M, Björkander S, Marchini G, Jenmalm MC, et al. Extreme prematurity and sepsis strongly influence frequencies and functional characteristics of circulating γδ T and natural killer cells. Clin Transl Immunol. 2021;10(6):e1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamdar S, Hutchinson R, Laing A, Stacey F, Ansbro K, Millar MR, et al. Perinatal inflammation influences but does not arrest rapid immune development in preterm babies. Nat Commun. 2020;11(1):1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Azizia M, Lloyd J, Allen M, Klein N, Peebles D. Immune status in very preterm neonates. Pediatrics. 2012;129(4):e967–74. [DOI] [PubMed] [Google Scholar]
- 11. Tissières P, Ochoda A, Dunn-Siegrist I, Drifte G, Morales M, Pfister R, et al. Innate immune deficiency of extremely premature neonates can be reversed by interferon-γ. PLoS One. 2012;7(3):e32863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Jong E, Strunk T, Burgner D, Lavoie PM, Currie A. The phenotype and function of preterm infant monocytes: implications for susceptibility to infection. J Leukoc Biol. 2017;102(3):645–56. [DOI] [PubMed] [Google Scholar]
- 13. Palojärvi A, Petäjä J, Siitonen S, Janér C, Andersson S. Low monocyte HLA-DR expression as an indicator of immunodepression in very low birth weight infants. Pediatr Res. 2013;73(4 Pt 1):469–75. [DOI] [PubMed] [Google Scholar]
- 14. Shen CM, Lin SC, Niu DM, Kou YR. Development of monocyte Toll-like receptor 2 and Toll-like receptor 4 in preterm newborns during the first few months of life. Pediatr Res. 2013;73(5):685–91. [DOI] [PubMed] [Google Scholar]
- 15. Marchant EA, Kan B, Sharma AA, van Zanten A, Kollmann TR, Brant R, et al. Attenuated innate immune defenses in very premature neonates during the neonatal period. Pediatr Res. 2015;78(5):492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schefold JC, Porz L, Uebe B, Poehlmann H, von Haehling S, Jung A, et al. Diminished HLA-DR expression on monocyte and dendritic cell subsets indicating impairment of cellular immunity in pre-term neonates: a prospective observational analysis. J Perinat Med. 2015;43(5):609–18. [DOI] [PubMed] [Google Scholar]
- 17. Eliwan HO, Watson WRG, Melo AM, Kelly LA, Omer M, Jafar A, et al. Selective modulation of monocyte and neutrophil responses with activated protein C in preterm infants. J Matern Fetal Neonatal Med. 2023;36(1):2183467. [DOI] [PubMed] [Google Scholar]
- 18. Strunk T, Hibbert J, Doherty D, Nathan E, Simmer K, Richmond P, et al. Impaired cytokine responses to live Staphylococcus epidermidis in preterm infants precede gram-positive, late-onset sepsis. Clin Infect Dis. 2021;72(2):271–8. [DOI] [PubMed] [Google Scholar]
- 19. Hibbert J, Strunk T, Nathan E, Prosser A, Doherty D, Simmer K, et al. Composition of early life leukocyte populations in preterm infants with and without late-onset sepsis. PLoS One. 2022;17(3):e0264768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hibbert J, Strunk T, Simmer K, Richmond P, Burgner D, Currie A. Plasma cytokine profiles in very preterm infants with late-onset sepsis. PLoS One. 2020;15(5):e0232933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donald K, Finlay BB. Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease. Nat Rev Immunol. 2023;23(11):735–48. [DOI] [PubMed] [Google Scholar]
- 22. Kolypetri P, Weiner HL. Monocyte regulation by gut microbial signals. Trends Microbiol. 2023;31(10):1044–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanchez-Schmitz G, Morrocchi E, Cooney M, Soni D, Khatun R, Palma P, et al. Neonatal monocytes demonstrate impaired homeostatic extravasation into a microphysiological human vascular model. Sci Rep. 2020;10(1):17836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562(7728):583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Healy DB, Ryan CA, Ross RP, Stanton C, Dempsey EM. Clinical implications of preterm infant gut microbiome development. Nat Microbiol. 2022;7(1):22–33. [DOI] [PubMed] [Google Scholar]
- 26. Korpela K, Blakstad EW, Moltu SJ, Strømmen K, Nakstad B, Rønnestad AE, et al. Intestinal microbiota development and gestational age in preterm neonates. Sci Rep. 2018;8(1):2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chernikova DA, Madan JC, Housman ML, Zain-Ul-Abideen M, Lundgren SN, Morrison HG, et al. The premature infant gut microbiome during the first 6 weeks of life differs based on gestational maturity at birth. Pediatr Res. 2018;84(1):71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martí M, Spreckels JE, Ranasinghe PD, Wejryd E, Marchini G, Sverremark-Ekström E, et al. Effects of Lactobacillus reuterisupplementation on the gut microbiota in extremely preterm infants in a randomized placebo-controlled trial. Cell Rep Med. 2021;2(3):100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van den Akker CHP, van Goudoever JB, Szajewska H, Embleton ND, Hojsak I, Reid D, et al. Probiotics for preterm infants: a strain-specific systematic review and network meta-analysis. J Pediatr Gastroenterol Nutr. 2018;67(1):103–22. [DOI] [PubMed] [Google Scholar]
- 30. Beck LC, Masi AC, Young GR, Vatanen T, Lamb CA, Smith R, et al. Strain-specific impacts of probiotics are a significant driver of gut microbiome development in very preterm infants. Nat Microbiol. 2022;7(10):1525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bargheet A, Klingenberg C, Esaiassen E, Hjerde E, Cavanagh JP, Bengtsson-Palme J, et al. Development of early life gut resistome and mobilome across gestational ages and microbiota-modifying treatments. EBioMedicine. 2023;92:104613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spreckels JE, Wejryd E, Marchini G, Jonsson B, de Vries DH, Jenmalm MC, et al. Lactobacillus reuteri colonisation of extremely preterm infants in a randomised placebo-controlled trial. Microorganisms. 2021;9(5):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wejryd E, Marchini G, Frimmel V, Jonsson B, Abrahamsson T. Probiotics promoted head growth in extremely low birthweight infants in a double-blind placebo-controlled trial. Acta Paediatr. 2019;108(1):62–9. [DOI] [PubMed] [Google Scholar]
- 34. Martí M, Spreckels JE, Jenmalm MC, Abrahamsson T. A protocol for characterization of extremely preterm infant gut microbiota in double-blind clinical trials. STAR Protoc. 2021;2(3):100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brooks B, Olm MR, Firek BA, Baker R, Geller-McGrath D, Reimer SR, et al. The developing premature infant gut microbiome is a major factor shaping the microbiome of neonatal intensive care unit rooms. Microbiome. 2018;6(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guevara MR, Hartmann D, Mendoza M. diverse: an R package to analyze diversity in complex systems. Radiokhimiya J. 2016;8(2):60–78. [Google Scholar]
- 37. Cao KAL, Rohart F, Gonzalez I, Sebastien D, Bartolo F, Bladen M, et al. Mix omics: omics data integration project. R Package Version 6.1.1. 2016.
- 38. Kennedy A, Waters E, Rowshanravan B, Hinze C, Williams C, Janman D, et al. Differences in CD80 and CD86 transendocytosis reveal CD86 as a key target for CTLA-4 immune regulation. Nat Immunol. 2022;23(9):1365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Halliday N, Williams C, Kennedy A, Waters E, Pesenacker AM, Soskic B, et al. CD86 is a selective CD28 ligand supporting FoxP3+ regulatory T cell homeostasis in the presence of high levels of CTLA-4. Front Immunol. 2020;11:600000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dilioglou S, Cruse JM, Lewis RE. Function of CD80 and CD86 on monocyte- and stem cell-derived dendritic cells. Exp Mol Pathol. 2003;75(3):217–27. [DOI] [PubMed] [Google Scholar]
- 41. Sharma AM, Birkett R, Lin ET, Ernst LM, Grobman WA, Swaminathan S, et al. Placental dysfunction influences fetal monocyte subpopulation gene expression in preterm birth. JCI Insight. 2022;7(11):e155482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nolan A, Weiden M, Kelly A, Hoshino Y, Hoshino S, Mehta N, et al. CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis. Am J Respir Crit Care Med. 2008;177(3):301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson J, Bender G, Minh Thang C, Quang Thanh L, Thi Trang Dai V, Van Thanh P, et al. TLR responses in preterm and term infant cord blood mononuclear cells. Pathogens. 2023;12(4):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekström ES, et al. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002;107(1):145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dubicke A, Andersson P, Fransson E, Andersson E, Sioutas A, Malmström A, et al. High-mobility group box protein 1 and its signalling receptors in human preterm and term cervix. J Reprod Immunol. 2010;84(1):86–94. [DOI] [PubMed] [Google Scholar]
- 46. Robertson SA, Hutchinson MR, Rice KC, Chin PY, Moldenhauer LM, Stark MJ, et al. Targeting Toll-like receptor-4 to tackle preterm birth and fetal inflammatory injury. Clin Transl Immunol. 2020;9(4):e1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schittenhelm L, Hilkens CM, Morrison VL. β2 integrins as regulators of dendritic cell, monocyte, and macrophage function. Front Immunol. 2017;8:1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Angelidou A, Diray-Arce J, Conti MG, Netea MG, Blok BA, Liu M, et al. Human newborn monocytes demonstrate distinct BCG-induced primary and trained innate cytokine production and metabolic activation in vitro. Front Immunol. 2021;12:674334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rohsiswatmo R, Azharry M, Sari TT, Bahasoan Y, Wulandari D. TLR2 and TLR4 expressions in late-onset neonatal sepsis: is it a potential novel biomarker? J Neonatal Perinatal Med. 2021;14(3):361–7. [DOI] [PubMed] [Google Scholar]
- 50. Turunen R, Andersson S, Nupponen I, Kautiainen H, Siitonen S, Repo H. Increased CD11b-density on circulating phagocytes as an early sign of late-onset sepsis in extremely low-birth-weight infants. Pediatr Res. 2005;57(2):270–5. [DOI] [PubMed] [Google Scholar]
- 51. Sharygin D, Koniaris LG, Wells C, Zimmers TA, Hamidi T. Role of CD14 in human disease. Immunology. 2023;169(3):260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Maldeghem I, Nusman CM, Visser DH. Soluble CD14 subtype (sCD14-ST) as biomarker in neonatal early-onset sepsis and late-onset sepsis: a systematic review and meta-analysis. BMC Immunol. 2019;20(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kuppala VS, Meinzen-Derr J, Morrow AL, Schibler KR. Prolonged initial empirical antibiotic treatment is associated with adverse outcomes in premature infants. J Pediatr. 2011;159(5):720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Drell T, Lutsar I, Stšepetova J, Parm U, Metsvaht T, Ilmoja ML, et al. The development of gut microbiota in critically ill extremely low birth weight infants assessed with 16S rRNA gene based sequencing. Gut Microbes. 2014;5(3):304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arboleya S, Sánchez B, Milani C, Duranti S, Solís G, Fernández N, et al. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538–44. [DOI] [PubMed] [Google Scholar]
- 56. Hikita N, Cho Y, Tachibana D, Hamazaki T, Koyama M, Tokuhara D. Cell surface antigens of neonatal monocytes are selectively impaired in basal expression, but hyperresponsive to lipopolysaccharide and zymosan. J Reprod Immunol. 2019;136:102614. [DOI] [PubMed] [Google Scholar]
- 57. Buechler C, Ritter M, Orsó E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67(1):97–103. [PubMed] [Google Scholar]
- 58. Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. 2011;187(7):3671–82. [DOI] [PubMed] [Google Scholar]
- 59. Austermann J, Roth J, Barczyk-Kahlert K. The good and the bad: monocytes’ and macrophages’ diverse functions in inflammation. Cells. 2022;11(12):1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yap YJ, Wong PF, AbuBakar S, Sam SS, Shunmugarajoo A, Soh YH, et al. The clinical utility of CD163 in viral diseases. Clin Chim Acta. 2023;541:117243. [DOI] [PubMed] [Google Scholar]
- 61. Svensson-Arvelund J, Mehta RB, Lindau R, Mirrasekhian E, Rodriguez-Martinez H, Berg G, et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol. 2015;194(4):1534–44. [DOI] [PubMed] [Google Scholar]
- 62. Hintz KA, Rassias AJ, Wardwell K, Moss ML, Morganelli PM, Pioli PA, et al. Endotoxin induces rapid metalloproteinase-mediated shedding followed by up-regulation of the monocyte hemoglobin scavenger receptor CD163. J Leukoc Biol. 2002;72(4):711–7. [PubMed] [Google Scholar]
- 63. Mata Forsberg M, Björkander S, Pang Y, Lundqvist L, Ndi M, Ott M, et al. Extracellular membrane vesicles from lactobacilli dampen IFN-γ responses in a monocyte-dependent manner. Sci Rep. 2019;9(1):17109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lasaviciute G, Barz M, van der Heiden M, Arasa C, Tariq K, Quin J, et al. Gut commensal Limosilactobacillus reuteri induces atypical memory-like phenotype in human dendritic cells in vitro. Gut Microbes. 2022;14(1):2045046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pang Y, Ermann Lundberg L, Mata Forsberg M, Ahl D, Bysell H, Pallin A, et al. Extracellular membrane vesicles from Limosilactobacillus reuteristrengthen the intestinal epithelial integrity, modulate cytokine responses and antagonize activation of TRPV1. Front Microbiol. 2022;13:1032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during the study period are included in the manuscript and the online supplementary materials. Further queries can be directed to the corresponding author.