Abstract
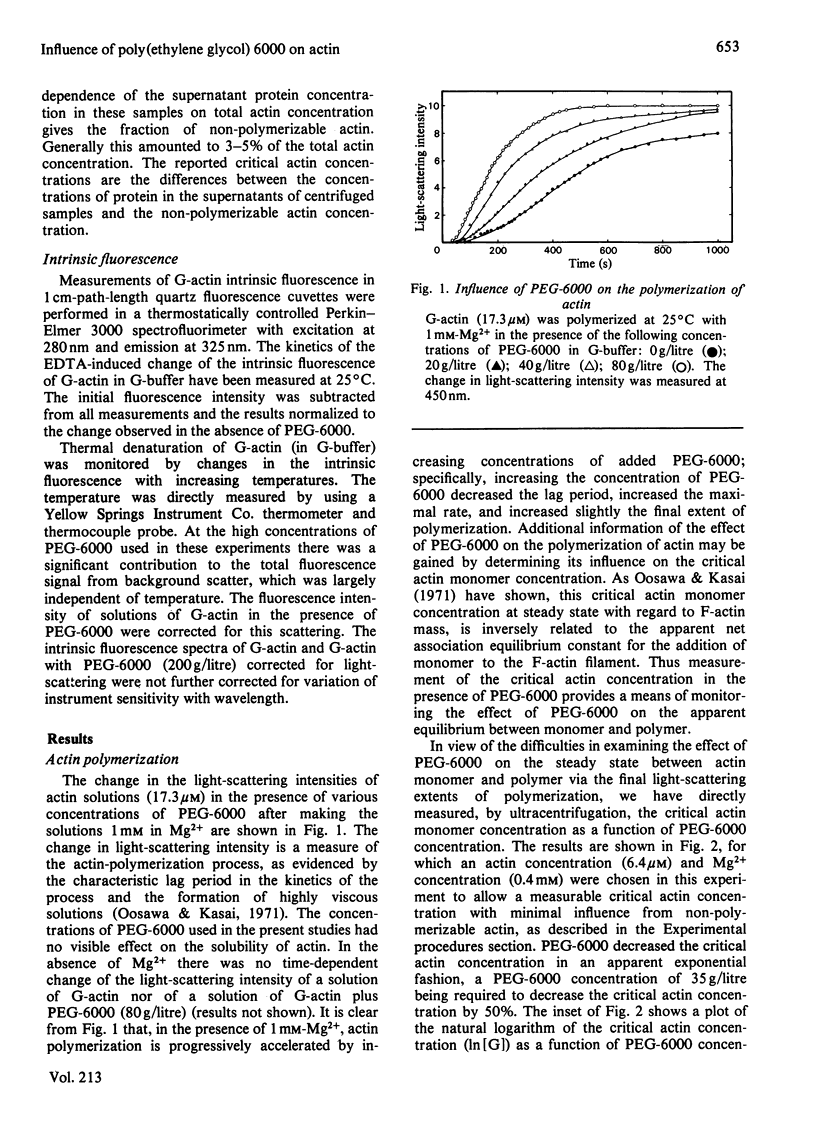
Poly(ethylene glycol) 6000 affected many of the properties of skeletal-muscle actin. It accelerated the rate and increased the extent of actin polymerization as measured by light-scattering and sedimentation studies respectively. Moreover, intrinsic-fluorescence measurements showed that addition of poly(ethylene glycol) 6000 decreased the rate of EDTA-induced denaturation of actin monomer and increased the temperature at which irreversible conformational changes occur in actin monomer. These effects occurred without any apparent direct binding interaction and are postulated to be a consequence of the effect of excluded volume on the thermodynamic activity of actin. A relationship based on spherical geometry was formulated which described the co-volume increment that occurs upon addition of a monomer to a long linear polymer in the presence of a space-filling macromolecule. The application of this relationship to the poly(ethylene glycol) 6000-actin system was not without assumption, but it permitted quantitative estimation of the co-volume increment which proved to be of the sign and magnitude required to explain the increased extent of actin polymerization found experimentally in the presence of various concentrations of poly(ethylene glycol) 6000. It is suggested that, in vivo, excluded volume may play a role in actin-filament formation and in the maintenance of the native G-actin structure.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Smith P. R., Isenberg G., Pollard T. D. Structure of crystalline actin sheets. Nature. 1980 Nov 20;288(5788):296–298. doi: 10.1038/288296a0. [DOI] [PubMed] [Google Scholar]
- Atha D. H., Ingham K. C. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J Biol Chem. 1981 Dec 10;256(23):12108–12117. [PubMed] [Google Scholar]
- Atha D. H., Ingham K. C. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J Biol Chem. 1981 Dec 10;256(23):12108–12117. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Busby T. F., Ingham K. C. Separation of macromolecules by ultrafiltration: removal of poly(ethylene glycol) from human albumin. J Biochem Biophys Methods. 1980 Apr;2(4):191–206. doi: 10.1016/0165-022x(80)90034-2. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Markey F., Blikstad I., Persson T., Lindberg U. Reorganization of actin in platelets stimulated by thrombin as measured by the DNase I inhibition assay. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6376–6380. doi: 10.1073/pnas.76.12.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Cooke R. The role of the bound nucleotide in the polymerization of actin. Biochemistry. 1975 Jul 15;14(14):3250–3256. doi: 10.1021/bi00685a035. [DOI] [PubMed] [Google Scholar]
- Detmers P., Weber A., Elzinga M., Stephens R. E. 7-Chloro-4-nitrobenzeno-2-oxa-1,3-diazole actin as a probe for actin polymerization. J Biol Chem. 1981 Jan 10;256(1):99–105. [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk T. W., Jr, Ue K. The measurement of actin concentration in solution: a comparison of methods. Anal Biochem. 1974 Nov;62(1):66–74. doi: 10.1016/0003-2697(74)90367-4. [DOI] [PubMed] [Google Scholar]
- KASAI M., ASAKURA S., OOSAWA F. The G-F equilibrium in actin solutions under various conditions. Biochim Biophys Acta. 1962 Feb 12;57:13–21. doi: 10.1016/0006-3002(62)91072-7. [DOI] [PubMed] [Google Scholar]
- KASAI M., ASAKURA S., OOSAWA F. The cooperative nature of G-F transformation of actin. Biochim Biophys Acta. 1962 Feb 12;57:22–31. doi: 10.1016/0006-3002(62)91073-9. [DOI] [PubMed] [Google Scholar]
- Kasai M. Thermodynamical aspect of G-F transformations of actin. Biochim Biophys Acta. 1969 Jun 24;180(2):399–409. doi: 10.1016/0005-2728(69)90124-8. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Maruyama K. Electron microscopic particle length of F-actin polymerized in vitro. J Biochem. 1970 Mar;67(3):437–457. doi: 10.1093/oxfordjournals.jbchem.a129267. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl W. M., Gergely J. The kinetics of exchange of adenosine triphosphate and calcium with G-actin. J Biol Chem. 1969 Sep 10;244(17):4720–4729. [PubMed] [Google Scholar]
- Lehrer S. S., Kerwar G. Intrinsic fluorescence of actin. Biochemistry. 1972 Mar 28;11(7):1211–1217. doi: 10.1021/bi00757a015. [DOI] [PubMed] [Google Scholar]
- MIHASHI K. MOLECULAR CHARACTERISTICS OF G-ADP ACTIN. Arch Biochem Biophys. 1964 Sep;107:441–448. doi: 10.1016/0003-9861(64)90300-5. [DOI] [PubMed] [Google Scholar]
- Nichol L. W., Ogston A. G., Wills P. R. Effect of inert polymers on protein self-association. FEBS Lett. 1981 Apr 6;126(1):18–20. doi: 10.1016/0014-5793(81)81022-8. [DOI] [PubMed] [Google Scholar]
- OOSAWA F., KASAI M. A theory of linear and helical aggregations of macromolecules. J Mol Biol. 1962 Jan;4:10–21. doi: 10.1016/s0022-2836(62)80112-0. [DOI] [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Mechanism of K+-induced actin assembly. J Cell Biol. 1982 Jun;93(3):648–654. doi: 10.1083/jcb.93.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D. Cytoskeletal functions of cytoplasmic contractile proteins. J Supramol Struct. 1976;5(3):317–334. doi: 10.1002/jss.400050306. [DOI] [PubMed] [Google Scholar]
- STEINER R. F., EDELHOCH H. Effect of thermally induced structural transitions on the ultra-violet fluorescence of proteins. Nature. 1962 Jan 27;193:375–376. doi: 10.1038/193375a0. [DOI] [PubMed] [Google Scholar]
- Strzelecka-Golaszewska H., Nagy B., Gergely J. Changes in conformation and nucleotide binding of Ca, Mn, or MgG-actin upon removal of the bound divalent cation. Studies of ultraviolet difference spectra and optical rotation. Arch Biochem Biophys. 1974 Apr 2;161(2):559–569. doi: 10.1016/0003-9861(74)90339-7. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Condeelis J. S. Cytoplasmic structure and contractility in amoeboid cells. Int Rev Cytol. 1979;56:57–144. doi: 10.1016/s0074-7696(08)61821-5. [DOI] [PubMed] [Google Scholar]
- Tellam R., Frieden C. Cytochalasin D and platelet gelsolin accelerate actin polymer formation. A model for regulation of the extent of actin polymer formation in vivo. Biochemistry. 1982 Jun 22;21(13):3207–3214. doi: 10.1021/bi00256a027. [DOI] [PubMed] [Google Scholar]
- Wegner A., Engel J. Kinetics of the cooperative association of actin to actin filaments. Biophys Chem. 1975 Jul;3(3):215–225. doi: 10.1016/0301-4622(75)80013-5. [DOI] [PubMed] [Google Scholar]
- Wills P. R., Nichol L. W., Siezen R. J. The indefinite self-association of lysozyme: consideration of composition-dependent activity coefficients. Biophys Chem. 1980 Feb;11(1):71–82. doi: 10.1016/0301-4622(80)85009-5. [DOI] [PubMed] [Google Scholar]


