Abstract
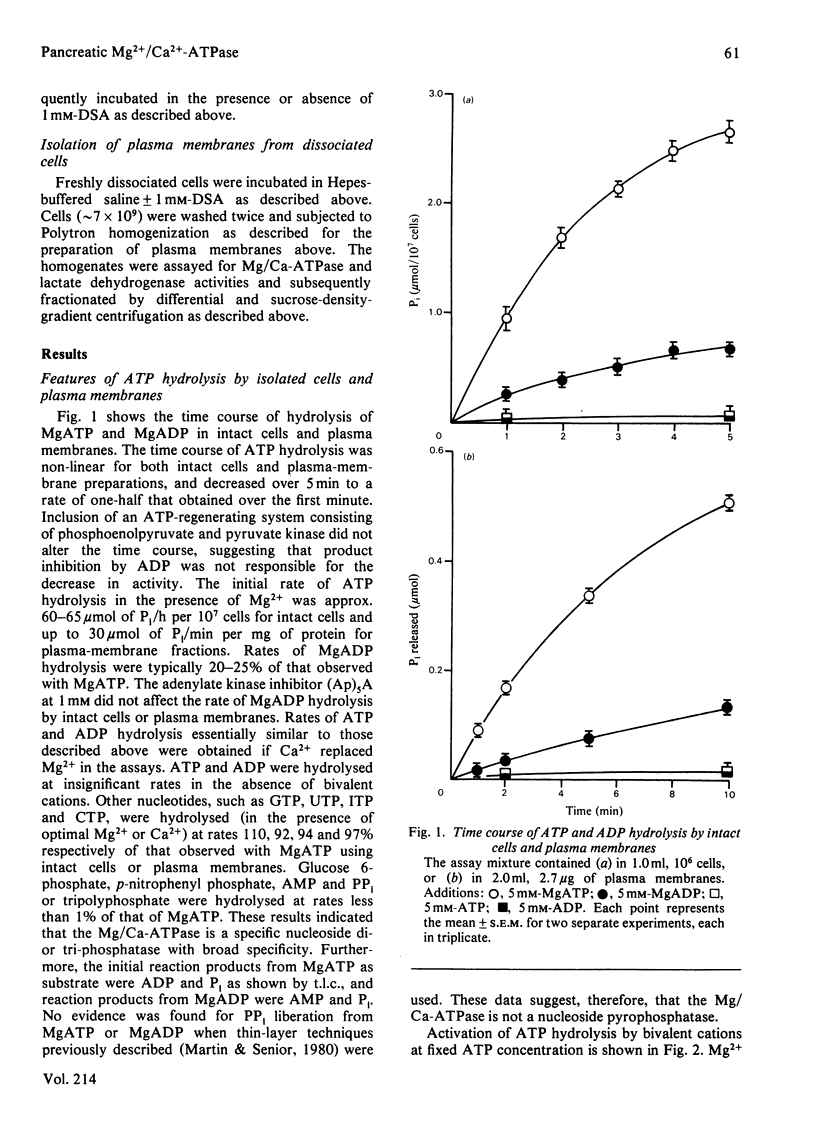
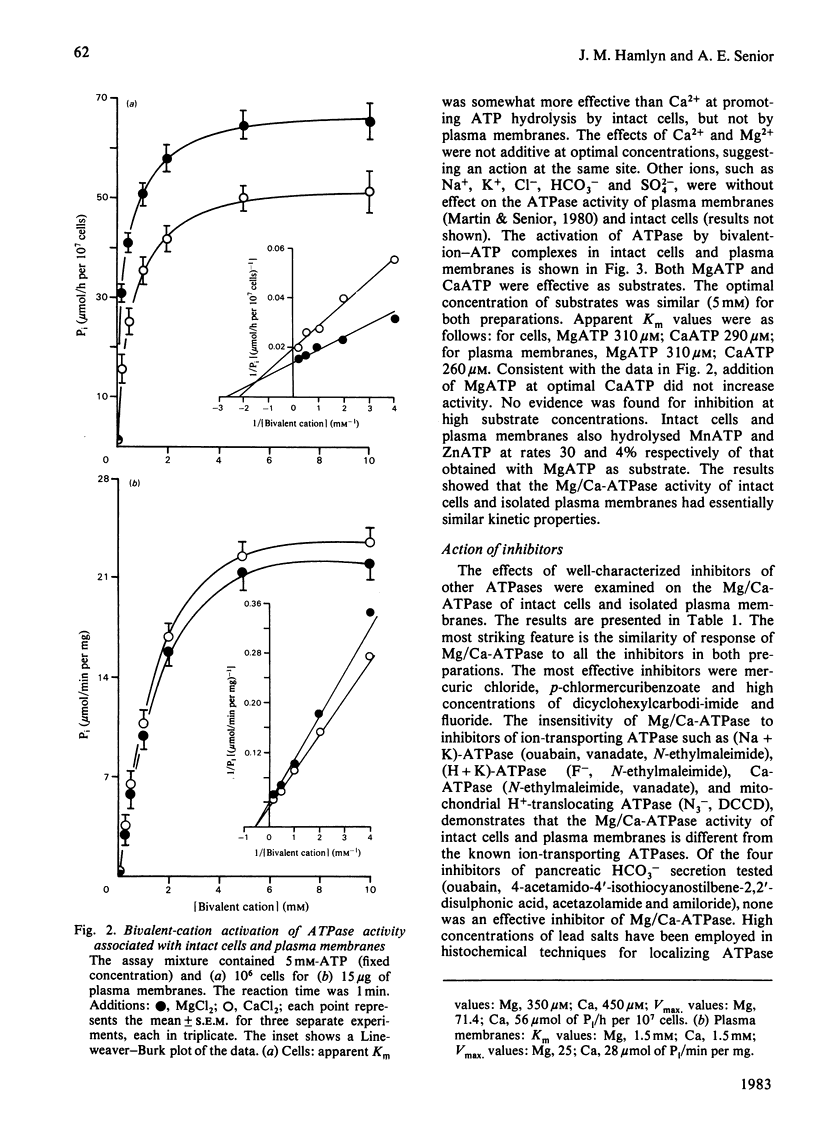
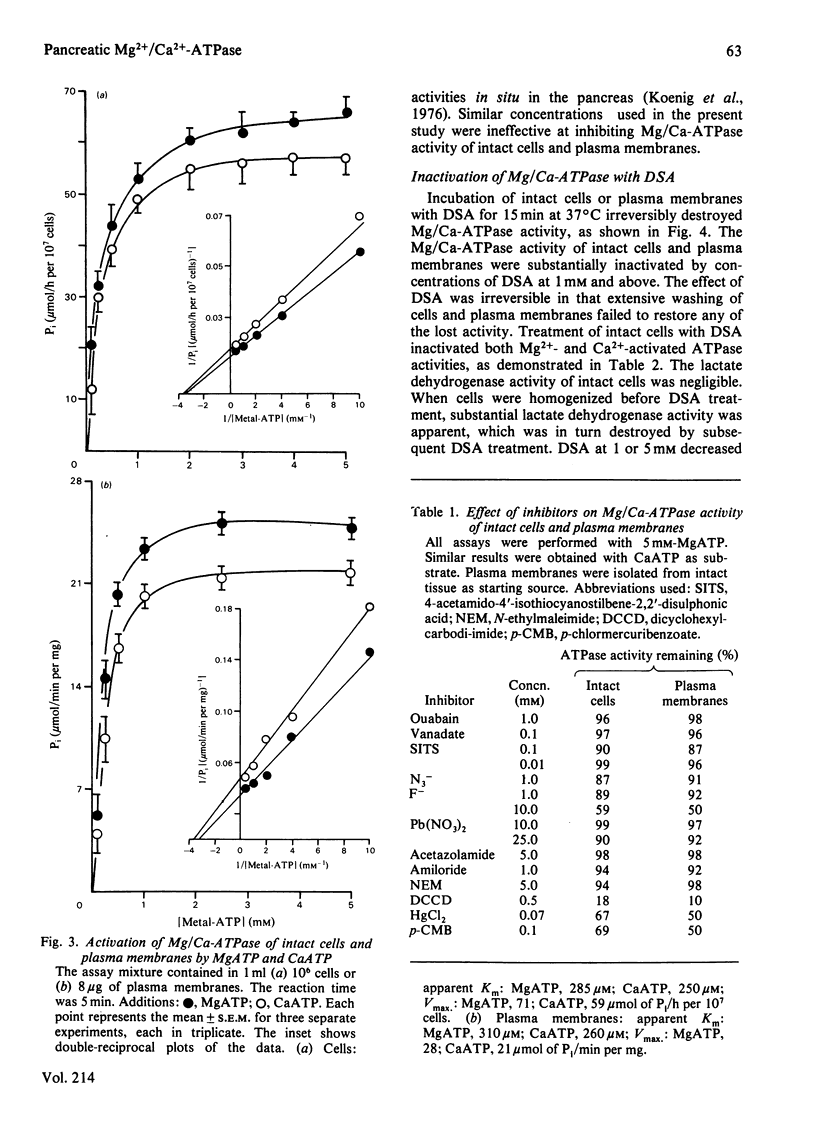
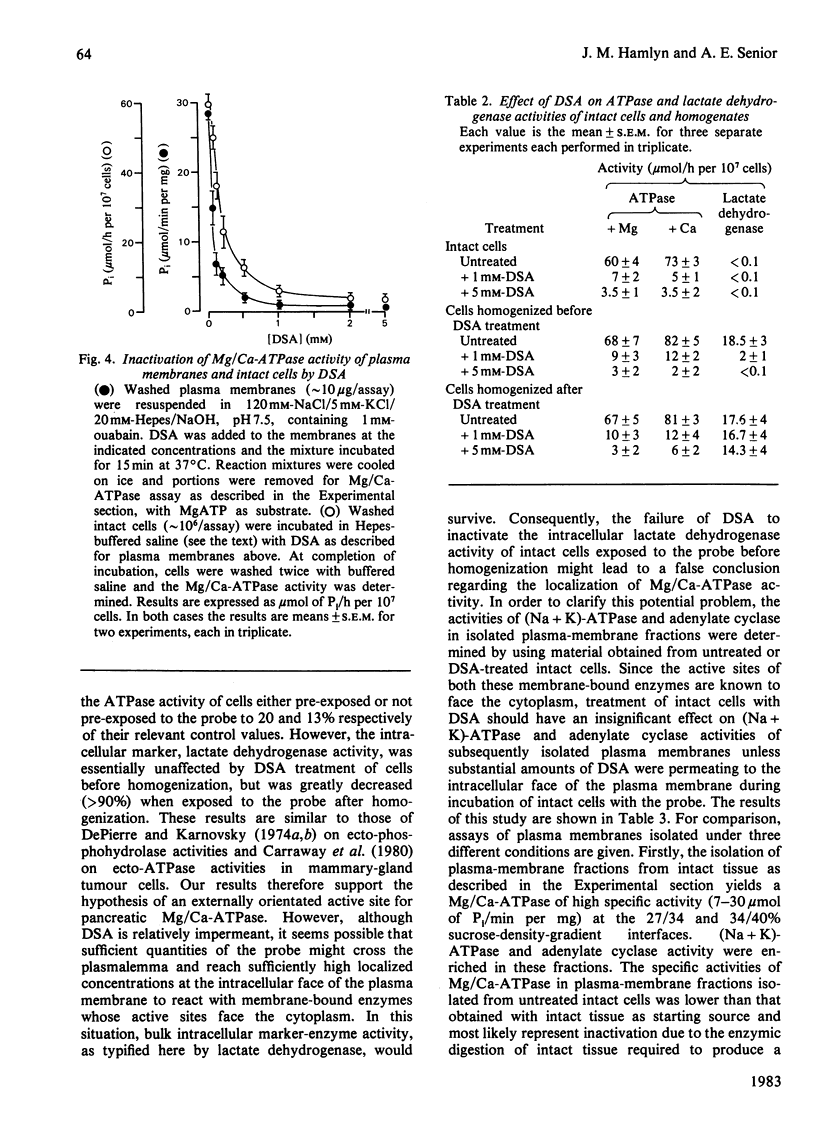
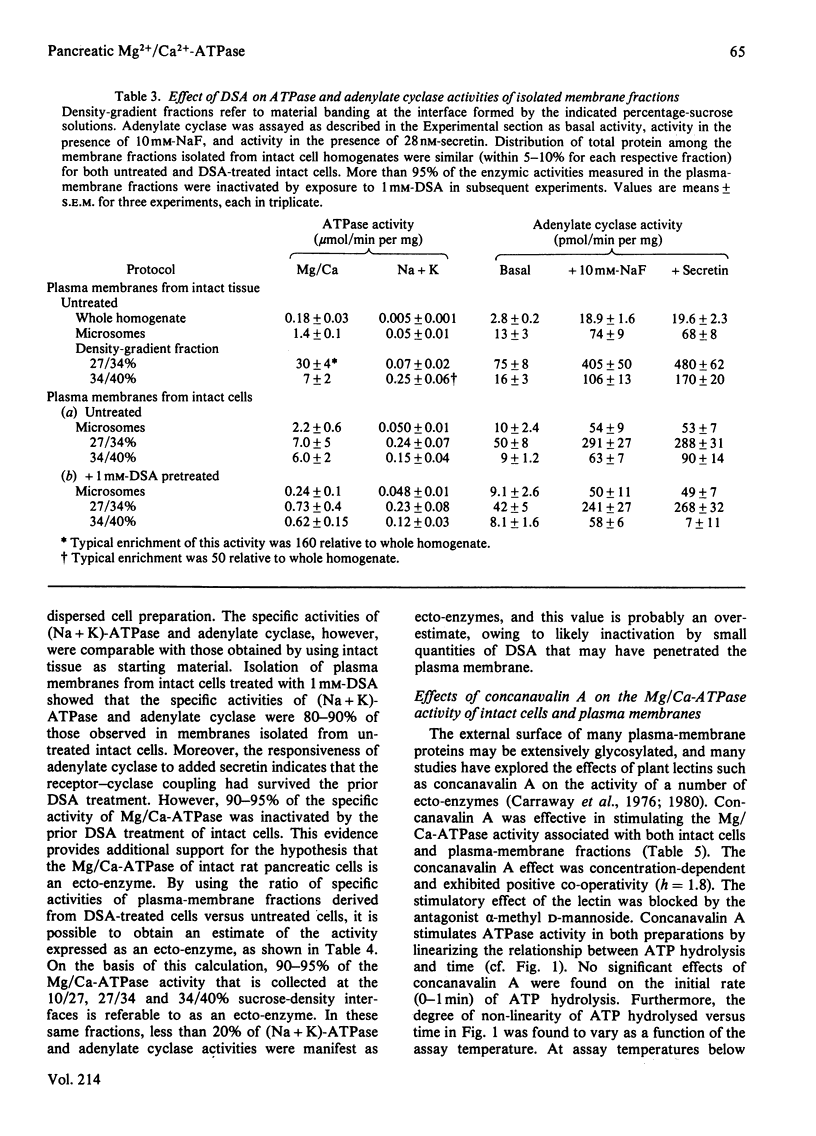
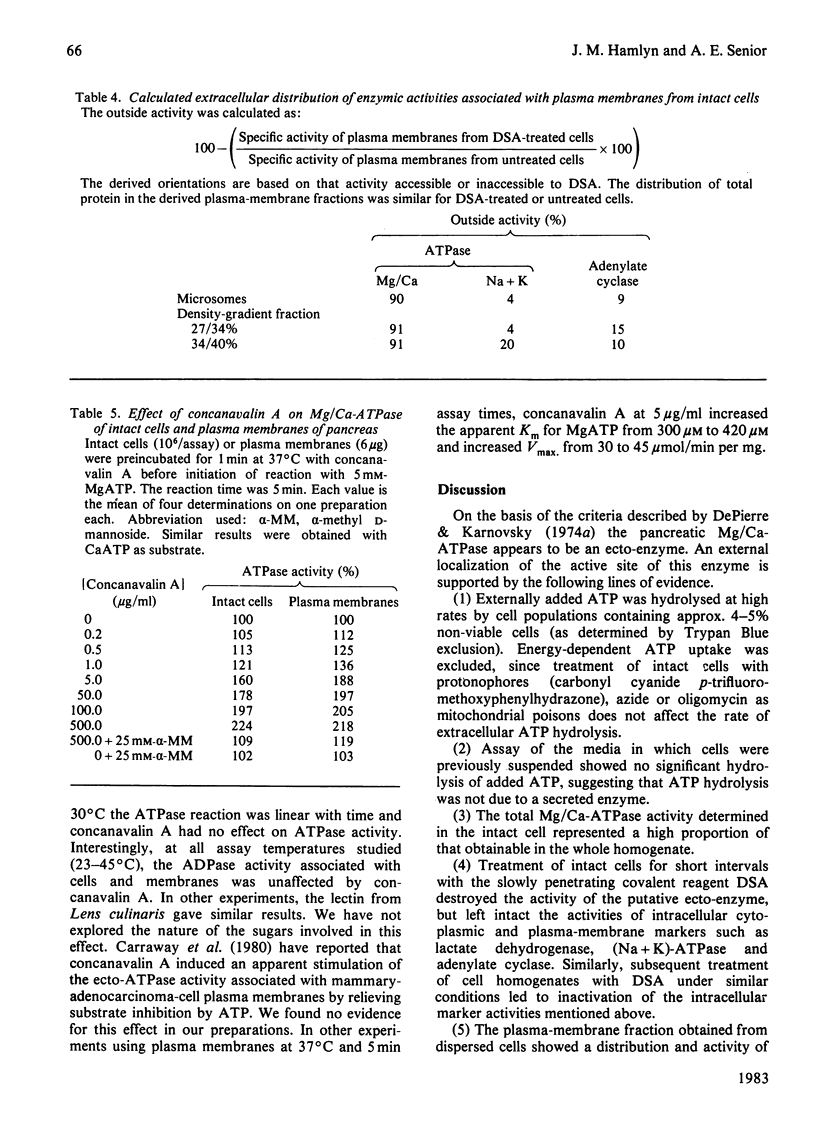
Preparations of enzymically dispersed rat pancreatic cells hydrolyse externally added nucleoside triphosphates and diphosphates at high rates in the presence of Mg2+ or Ca2+. The lack of response to specific inhibitors and activators differentiates this hydrolytic activity from that of other well-characterized ion-transporting ATPases. Studies based on inactivation of this hydrolytic activity by the covalently reacting, slowly permeating probe diazotized sulphanilic acid indicated that this nucleoside tri- and di-phosphatase is primarily a plasma-membrane ecto-enzyme. It is the major ATPase activity associated with intact cells, homogenates and isolated plasma-membrane fractions. Concanavalin A stimulates this ATPase activity of intact cells and isolated plasma-membrane fractions. The insensitivity of this ATPase activity to univalent ions and inhibitors of pancreatic electrolyte secretion, taken together with the evidence that the active site is externally located, suggests that this enzyme is not directly involved in HCO3- secretion in the pancreas. Its actual function remains unknown.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Jamieson J. D. Studies on dispersed pancreatic exocrine cells. I. Dissociation technique and morphologic characteristics of separated cells. J Cell Biol. 1974 Dec;63(3):1037–1056. doi: 10.1083/jcb.63.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashear R. E., Ross J. C., Smith C. N. Rest and exercise plasms nucleotides. Respir Physiol. 1968 Oct;5(3):401–406. doi: 10.1016/0034-5687(68)90031-5. [DOI] [PubMed] [Google Scholar]
- Carraway C. A., Corrado F. J., 4th, Fogle D. D., Carraway K. L. Ecto-enzymes of mammary gland and its tumours. Ca2+- or Mg2+-stimulated adenosine triphosphatase and its perturbation by concanavalin A. Biochem J. 1980 Oct 1;191(1):45–51. doi: 10.1042/bj1910045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway K. L., Fogle D. D., Chestnut R. W., Huggins J. W., Carraway C. A. Ecto-enzymes of mammary gland and its tumors. Lectin inhibition of 5'-nucleotidase of the 13762 rat mammary ascites carcinoma. J Biol Chem. 1976 Oct 25;251(20):6173–6178. [PubMed] [Google Scholar]
- Chambers D. A., Salzman E. W., Neri L. L. Characterization of "ecto-ATPase" of human blood platelets. Arch Biochem Biophys. 1967 Mar;119(1):173–178. doi: 10.1016/0003-9861(67)90444-4. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. I. Evidence for an ecto-adenosine monophosphatase, adenosine triphosphatase, and -p-nitrophenyl phosphates. J Biol Chem. 1974 Nov 25;249(22):7111–7120. [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. II. Properties and suitability as markers for the plasma membrane. J Biol Chem. 1974 Nov 25;249(22):7121–7129. [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T. The identification of adenosine triphosphate in fresh human plasma. J Physiol. 1969 Jan;200(1):53P–54P. [PubMed] [Google Scholar]
- Gallacher D. V. Are there purinergic receptors on parotid acinar cells? Nature. 1982 Mar 4;296(5852):83–86. doi: 10.1038/296083a0. [DOI] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig C. S., Santelices L. C., Vial J. D. Cytochemical study of the distribution of adenosine triphosphatase in the pancreas of the dog. J Histochem Cytochem. 1976 Oct;24(10):1065–1075. doi: 10.1177/24.10.135806. [DOI] [PubMed] [Google Scholar]
- Martin S. S., Senior A. E. Membrane adenosine triphosphatase activities in rat pancreas. Biochim Biophys Acta. 1980 Nov 4;602(2):401–418. doi: 10.1016/0005-2736(80)90320-x. [DOI] [PubMed] [Google Scholar]
- Poirier G. G., Lambert M. P., Lebel D., Sakr F., Morisset J., Beaudoin A. R. Purification of plasma membranes from rat pancreas: a rapid method. Proc Soc Exp Biol Med. 1977 Jul;155(3):324–329. doi: 10.3181/00379727-155-39799. [DOI] [PubMed] [Google Scholar]
- Ronquist G., Agren G. K. A Mg2+- and Ca2+-stimulated adenosine triphosphatase at the outer surface of Ehrlich ascites tumor cells. Cancer Res. 1975 Jun;35(6):1402–1406. [PubMed] [Google Scholar]
- Rorive G., Kleinzeller A. The effect of ATP and Ca 2+ on the cell volume in isolated kidney tubules. Biochim Biophys Acta. 1972 Jul 3;274(1):226–239. doi: 10.1016/0005-2736(72)90296-9. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. A Specific effect of external ATP on the permeability of transformed 3T3 cells. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1581–1588. doi: 10.1016/0006-291x(75)90207-7. [DOI] [PubMed] [Google Scholar]
- Schuurmans Stekhoven F., Bonting S. L. Transport adenosine triphosphatases: properties and functions. Physiol Rev. 1981 Jan;61(1):1–76. doi: 10.1152/physrev.1981.61.1.1. [DOI] [PubMed] [Google Scholar]
- Simon B., Kinne R., Sachs G. The presence of a HCO 3 - -ATPase in pancreatic tissue. Biochim Biophys Acta. 1972 Sep 1;282(1):293–300. doi: 10.1016/0005-2736(72)90335-5. [DOI] [PubMed] [Google Scholar]
- Simon B., Thomas L. HCO 3 -stimulated ATPase from mammalian pancreas. Properties and its arrangement with other enzyme activities. Biochim Biophys Acta. 1972 Nov 2;288(2):434–442. doi: 10.1016/0005-2736(72)90264-7. [DOI] [PubMed] [Google Scholar]
- Smolen J. E., Weissmann G. Mg2+-ATPase as a membrane ecto-enzyme of human granulocytes. Inhibitors, activators and response to phagocytosis. Biochim Biophys Acta. 1978 Oct 4;512(3):525–538. doi: 10.1016/0005-2736(78)90162-1. [DOI] [PubMed] [Google Scholar]
- Stefanovic V., Ciesielski-Treska J., Ebel A., Mandel P. Neuroblasts-glia interaction. The effect of co-cultivation upon ecto-ATPase activity of neuroblastoma and glioma cells. Exp Cell Res. 1976 Mar 1;98(1):191–203. doi: 10.1016/0014-4827(76)90479-1. [DOI] [PubMed] [Google Scholar]
- Swanson C. H., Solomon A. K. A micropuncture investigation of the whole tissue mechanism of electrolyte secretion by the in vitro rabbit pancreas. J Gen Physiol. 1973 Oct;62(4):407–429. doi: 10.1085/jgp.62.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G. Evidence for ATP action on the cell surface. Nature. 1974 Dec 6;252(5483):480–482. doi: 10.1038/252480a0. [DOI] [PubMed] [Google Scholar]
- Trams E. G., Lauter C. J. On the sidedness of plasma membrane enzymes. Biochim Biophys Acta. 1974 Apr 29;345(2):180–197. doi: 10.1016/0005-2736(74)90257-0. [DOI] [PubMed] [Google Scholar]
- WALLACH D. F., ULLREY D. The hydrolysis of ATP and related nucleotides by Ehrlich ascites carcinoma cells. Cancer Res. 1962 Feb;22:228–234. [PubMed] [Google Scholar]
- Wizemann V., Christian A. L., Wiechmann J., Schulz I. The distribution of membrane bound enzymes in the acini and ducts of the cat pancreas. Pflugers Arch. 1974 Feb 18;347(1):39–47. doi: 10.1007/BF00587053. [DOI] [PubMed] [Google Scholar]
- van Amelsvoort J. M., Jansen J. W., De Pont J. J., Bonting S. L. Is there a plasma membrane-located anion-sensitive ATPase? IV. Distribution of the enzyme in rat pancreas. Biochim Biophys Acta. 1978 Sep 22;512(2):296–308. doi: 10.1016/0005-2736(78)90254-7. [DOI] [PubMed] [Google Scholar]


