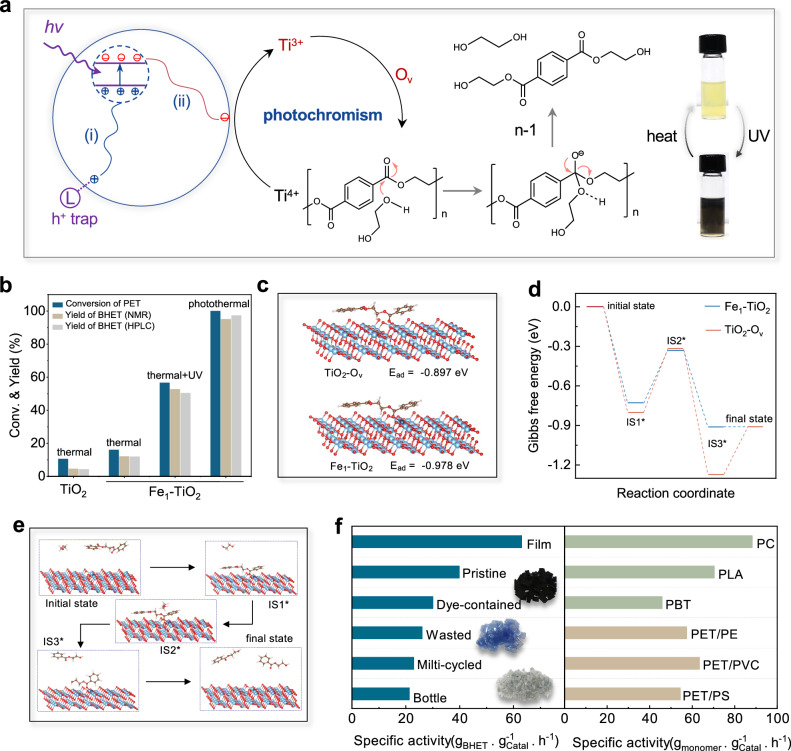
Fig. 5. Photothermal catalysis.
a Photochromism mechanism and photothermal catalytic processes of polyethylene terephthalate (PET) glycolysis. b PET conversion and bis(2-hydroxyethyl) terephthalate (BHET) yield at various conditions. In thermal and thermal + UV conditions the reactor was placed in an oil bath to keep a temperature of 190 oC. The UV light intensity is 0.052 W·cm‒2. In photothermal, the reactor was irradiation by simulated sunlight (0.52 W·cm‒2). c The adsorbed model and adsorption energy of ethylene glycol dibenzoate (EGD) on TiO2-Ov and Fe1-TiO2 slabs. Atom key: Ti (light blue), Fe (navy), O (red), C (green), H (white). d, e The calculated Gibbs free energy profiles and the intermediate models of photothermal catalytic PET glycolysis on TiO2-Ov and Fe1-TiO2 slabs. Atom key: Ti (light blue), Fe (navy), O (red), C (green), H (white). f The specific activities of Fe1-TiO2 toward various polyesters. Insets are the images of dyed PET lunch boxes (black), PET bottles (blue), and multi-cycled PET flakes (gray). About 20 mg of Fe1-TiO2 catalysts, 2 g of ethylene glycol, and 0.5 g of various polyester flakes were added to a sealed quartz reactor with a thermocouple inserted to keep a temperature of 190 oC. Source data are provided as a Source Data file.