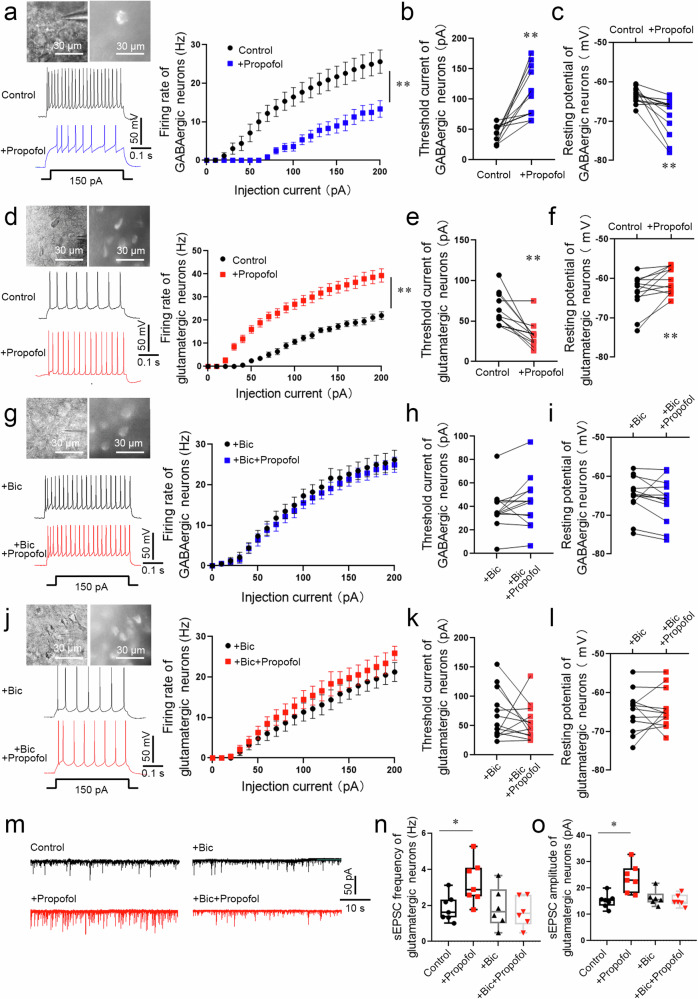
Fig. 3. The excitability of glutamatergic and GABAergic neurons in the BLA was enhanced or attenuated, respectively, by propofol anesthesia after FC training in mice.
a The electrophysiology of GABAergic (mCherry+) neurons in the BLA of Vgat-cre mice. A typical trace of membrane potential observed before and after the application of propofol (5 µM). Firing rate of action potentials evoked by depolarizing current pulses of 0–200 pA, values were presented (n = 13 neurons from 6 mice, two-way repeated-measures ANOVA, F (20, 504) = 2.936, p < 0.0001, **p < 0.01 vs. Control group). Threshold currents that evoked the first action potential (b) and comparison of resting membrane potential (c), (n = 13 neurons from 6 mice, two-tailed paired t test and two-tailed Wilcoxon matched-pairs signed rank test, **p < 0.01). d The electrophysiology of glutamatergic neurons (mCherry+) in the BLA of Vglut2-cre mice. A typical trace of membrane potential observed before and after the application of propofol (5 µM). Firing rate of action potentials evoked by depolarizing current pulses of 0–200 pA, values were presented (n = 13 neurons from 6 mice, two-way repeated-measures ANOVA, F (20, 502) = 5.073, p < 0.0001, **p < 0.01). e, f Threshold currents that evoked the first action potential and comparison of resting membrane potential, (n = 13 neurons from 6 mice, two-tailed Wilcoxon matched-pairs signed rank test, **p < 0.01). g The electrophysiology of GABAergic neurons (mCherry+) perfused bicuculline (30 µM) in the BLA of Vgat-cre mice. A typical trace of membrane potential observed before and after the application of propofol (5 µM). Firing rate of action potentials evoked by depolarizing current pulses of 0–200 pA, values were presented (n = 13 neurons from 6 mice, two-way repeated-measures ANOVA, F (20, 504) = 0.076, p > 0.999). h Threshold currents that evoked the first action potential, values were presented (n = 13 neurons from 6 mice, two-tailed Wilcoxon matched-pairs signed rank test, p > 0.05). i Comparison of resting membrane potential (n = 13 neurons from 6 mice, two-tailed paired t test, p > 0.05). j The electrophysiology of glutamatergic neurons (mCherry+) perfused bicuculline (30 µM) in the BLA of Vglut2-cre mice. A typical trace of membrane potential observed before and after the application of propofol (5 µM). Firing rate of action potentials evoked by depolarizing current pulses of 0–200 pA, values were presented (n = 13 neurons from 6 mice, two-way repeated-measures ANOVA, F (20, 504) = 0.212, p > 0.999). k Threshold currents that evoked the first action potential, values were presented (n = 13 neurons from 6 mice, two-tailed paired t test, p > 0.05). l Comparison of resting membrane potential (n = 13 neurons from 6 mice, two-tailed Wilcoxon matched-pairs signed rank test, p > 0.05). m Representative traces of sEPSC from glutamatergic neurons (mCherry+) in the BLA of Vglut2-cre mice. Scale bars = 50 pA, 10 s. n The sEPSC frequency in glutamatergic neurons was significantly increased in brain slices perfused propofol (5 µM) compared with those from control (n = 7 neurons from 6 mice, two-tailed paired t test, *p = 0.032). While The sEPSC frequency showed no statistical difference in brain slices perfused bicuculline + propofol and slices perfused bicuculline (n = 6 neurons from 6 mice, two-tailed paired t test, p > 0.05). o The sEPSC amplitude in glutamatergic neurons was significantly increased in brain slices perfused propofol (5 µM) compared with those from control (n = 7 neurons from 6 mice, two-tailed paired t test, *p = 0.013). While The sEPSC amplitude showed no statistical difference in brain slices perfused bicuculline + propofol and slices perfused bicuculline (n = 6 neurons from 6 mice, two-tailed paired t test, p > 0.05). All data are presented as the mean ± SEM.