Abstract
Multistep phosphorelay (MSP) signaling integrates hormonal and environmental signals to control both plant development and adaptive responses. Type-A RESPONSE REGULATOR (RRA) genes, the downstream members of the MSP cascade and cytokinin primary response genes, are thought to mediate primarily the negative feedback regulation of (cytokinin-induced) MSP signaling. However, transcriptional data also suggest the involvement of RRA genes in stress-related responses. By employing evolutionary conservation with the well-characterized Arabidopsis thaliana RRA genes, we identified five and 38 novel putative RRA genes in Brassica oleracea and Brassica napus, respectively. Our phylogenetic analysis suggests the existence of gene-specific selective pressure, maintaining the homologs of ARR3, ARR6, and ARR16 as singletons during the evolution of Brassicaceae. We categorized RRA genes based on the kinetics of their cytokinin-mediated up-regulation and observed both similarities and specificities in this type of response across Brassicaceae species. Using bioinformatic analysis and experimental data demonstrating the cytokinin and abiotic stress responsiveness of the A. thaliana-derived TCSv2 reporter, we unveil the mechanistic conservation of cytokinin- and stress-mediated up-regulation of RRA genes in B. rapa and B. napus. Notably, we identify partial cytokinin dependency of cold stress-induced RRA transcription, thus further demonstrating the role of cytokinin signaling in crop adaptive responses.
Keywords: Arabidopsis thaliana, Brassica napus, Brassica oleracea, Brassica rapa, cytokinins, multistep phosphorelay, osmotic stress, salinity, two-component signaling, type-A response regulator
We identified Brassica napus homologs of Arabidopsis thaliana type-A response regulator (RRA ) genes, and demonstrated the existence of selective pressure preventing their multiplication during Brassicaceae evolution, and the cytokinin dependency of their cold-induced up-regulation.
Introduction
Cytokinins regulate a wide range of biological processes that are vital for plant growth and development (Werner and Schmulling, 2009; Zurcher and Muller, 2016; Cortleven et al., 2019). In Arabidopsis thaliana, cytokinin signaling occurs through a multistep phosphorelay (MSP), sometimes also called two-component signaling (Kieber and Schaller, 2018). The core components of MSP include ARABIDOPSIS HISTIDINE KINASEs (AHKs), ARABIDOPSIS HISTIDINE-CONTAINING PHOSPHOTRANSMITTERs (AHPs), and ARABIDOPSIS RESPONSE REGULATORs (ARRs). In the presence of cytokinins, the CHASE-containing AHKs (AHK2, AHK3, and AHK4) located at the plasma membrane or endoplasmic reticulum (ER) undergo autophosphorylation at a conserved His residue and transfer the phosphate group to the conserved Asp residue within the AHK receiver domain (Hwang and Sheen, 2001; Inoue et al., 2001; Muller and Sheen, 2007; Antoniadi et al., 2020; Kubiasova et al., 2020). Cytoplasmic AHPs accept the phosphate from the AHKs and translocate to the nucleus, allowing the final transphosphorylation of the receiver domain of type-B RRs (RRBs) and transcriptional regulation of the cytokinin-responsive genes.
In addition to the aforementioned RRBs, the A. thaliana genome contains two more types of RRs: type-A RRs (RRAs) and type-C RRs (RRCs; Imamura et al., 1998; Schaller et al., 2008). RRBs possess a cytokinin-responsive receiver domain along with a large C-terminal extension that harbors the GARP (Golden/ARR/Psr1) motif, a Myb-like DNA-binding domain (Hosoda et al., 2002). In contrast, the RRAs are characterized by the presence of a receiver domain and short C-terminal sequences but do not contain the DNA-binding domain. RRA genes act as cytokinin primary response genes, being rapidly induced by cytokinins via direct transcriptional activation by RRBs, even in the absence of de novo protein synthesis (Taniguchi et al., 1998; D’Agostino et al., 2000). RRA proteins are phosphorylated by RRBs and mediate the negative regulation of MSP signaling via as yet unknown mechanisms (Lee et al., 2008). There are 10 known RRA genes in A. thaliana (ARR3, ARR4, ARR5, ARR6, ARR7, ARR8, ARR9, ARR15, ARR16, and ARR17), acting as partially redundant negative regulators of (cytokinin-induced) MSP signaling (To et al., 2004).
Previous studies have demonstrated the key role of A. thaliana RRA genes in several developmental and growth regulatory processes including stem cell specification, meristem activity, and regeneration (Leibfried et al., 2005; Muller and Sheen, 2008; Buechel et al., 2010; Zhao et al., 2010). In addition, the transcriptional activity of RRA genes was shown to be linked to diverse abiotic stress responses, including salinity, cold, and drought (Urao et al., 1998; Jain et al., 2006; Tran et al., 2007; Jeon et al., 2010; Kang et al., 2012; Shi et al., 2012; Sharan et al., 2017; Wang et al., 2019; Bhaskar et al., 2021). For instance, exposure to cold and dehydration stress triggers the up-regulation of ARR5, ARR6, ARR7, and ARR15. These RRA genes were shown to play a negative role in cold and dehydration stress regulation in A. thaliana (Jeon et al., 2010; Kang et al., 2012). Furthermore, overexpression of the rice RRA gene OsRR6 increased drought and salinity tolerance in A. thaliana (Bhaskar et al., 2021). All the aforementioned findings suggest the important role of RRA genes in abiotic stress responses. However, the role of cytokinins and/or cytokinin signaling in the regulation of stress-mediated up-regulation of RRA genes is not clear.
Advancements in sequencing technologies have facilitated the genome-wide identification of putative components of the MSP cascade not only in A. thaliana (Hwang and Sheen, 2001) but also in crop species such as rice (Ito and Kurata, 2006; Jain et al., 2006; Pareek et al., 2006; Karan et al., 2009; Tsai et al., 2012; Sharan et al., 2017), maize (Asakura et al., 2003), soybean (Mochida et al., 2010), and wheat (Sun et al., 2022). Members of the Brassica family are among the most commercially valuable species, as both culinary vegetables and oilseed crops, covering ~38 Mha globally (Kumar et al., 2009; European Commission, 2019; Rathore et al., 2022). Several genes involved in MSP signaling have been reported in Chinese cabbage [B. rapa spp. Pekinensis (Liu et al., 2014; Kaltenegger et al., 2018)], B. oleracea (Kaltenegger et al., 2018), and B. napus (Kuderova et al., 2015; Jiang et al., 2022). However, a comprehensive characterization of RRA genes and their orthologs across important crop species is lacking within the current scientific literature. Considering the transcriptional activation of RRA genes as a dynamic readout of nearly immediate changes in MSP activity (D’Agostino et al., 2000; Hejatko et al., 2009; Pernisova et al., 2009), this represents a substantial gap in our understanding of the role of MSP signaling in the control of plant development and adaptive responses.
In this study, we identify novel RRA genes in B. napus and B. oleracea and provide insights into the evolutionary relationships, kinetics, and mechanism of cytokinin responses, as well as the involvement of cytokinin in the abiotic stress-mediated modulation of RRA genes within A. thaliana and Brassica species.
Materials and methods
Identification of type-A response regulators in Brassica species, motif search, multiple sequence alignment, and chromosomal mapping
The protein sequences of the 10 known type-A RRs in the Arabidopsis thaliana genome (Hwang et al., 2002) were obtained from NCBI (https://www.ncbi.nlm.nih.gov/protein/) (NCBI reference sequence ARR3 NP_176202.1, ARR4 NP_001321924.1, ARR5 NP_190393.1, ARR6 NP_201097.1, ARR7 NP_173339.1, ARR8 NP_181663.1, ARR9 NP_001325622.1, ARR15 NP_177627.1, ARR16 NP_181599.1, and ARR17 NP_567037.1) (Supplementary Table S1). These sequences were used as queries in Protein BLAST (BLASTP) searches against the protein database of B. oleracea, B. rapa, and B. napus in EnsemblPlants (Release 51) (Howe et al., 2021). Genes were selected as described by Kaltenegger et al. (2018). The coding sequences, genomic sequences, and protein sequences of the selected genes were retrieved from EnsemblPlants (Release 51) (Howe et al., 2021) and Brassicaceae Database (BRAD version 3.0; http://brassicadb.cn) (Chen et al., 2021).
Using the Expasy SIM-Alignment Tool for protein sequences with BLOSUM62 as a comparison matrix (https://web.expasy.org/sim/) (Duvaud et al., 2021), the amino acid sequence homology of the identified Brassica RRAs was compared with A. thaliana RRAs (Supplementary Table S2). Similarly, the B. napus RRA genes from both A and C subgenomes were compared with those of their progenitor species B. rapa and B. oleracea. The presence of the conserved response regulator domain was analyzed using the GenomeNet Bioinformatics Tools, sequence motif search, MOTIF (https://www.genome.jp/tools/motif/) of Kyoto University Bioinformatics Center. The protein sequences of the identified Brassica RRA genes were used as input, and a search against the PFAM database was performed with a cut-off score of E-value=1. Sequences that possessed the conserved response regulator receiver (Rec) domain (PF00072) were selected for further analysis in this study.
Multiple sequence alignment was conducted using the MUSCLE algorithm (Edgar, 2004) implemented in UGENE (Okonechnikov et al., 2012) to annotate the location of important conserved residues. The genomic locations of A. thaliana and Brassica RRA genes were retrieved from EnsemblPlants (Release 51) (Howe et al., 2021) and BrassicaDB (BRAD version 3.0; http://brassicadb.cn) databases (Chen et al., 2021). These locations were visualized using MapGene2Chrom (MG2C_v2.1, http://mg2c.iask.in/mg2c_v2.1/) (Chao et al., 2015) by setting appropriate parameters for the figure output. The identified Brassica RRA genes were named following the nomenclature proposed by Heyl et al. (2013), and the numbers assigned to them correspond to their A. thaliana counterparts after performing phylogenetic analysis. In cases where multiple homologs of ARR4, ARR5, ARR7, ARR8, ARR9, ARR15, and ARR17 were found in Brassica, they were designated with the letters ‘a’, ‘b’, or ‘c’ in descending order of homology depending on the percentage amino acid identities they share with that specific RRA.
Phylogenetic analysis of type-A response regulator genes and gene structure analysis
A comparative phylogenetic analysis was conducted using MEGA7 (Kumar et al., 2016) based on the alignment of the conserved Rec domain (PF00072) as described by Kaltenegger et al. (2018). The multiple sequence alignment was performed using the conserved Rec domain employing the MUSCLE algorithm (Edgar, 2004) implemented in MEGA7 (Kumar et al., 2016). The Neighbor–Joining method (Saitou and Nei, 1987) was used to infer the evolutionary history. The evolutionary distances were computed using the Poisson correction method (Zuckerkandl and Pauling, 1965) and are expressed as the number of amino acid substitutions per site. The analysis included 1000 bootstrap replicates, and all ambiguous positions were removed for each sequence pair. Phylogenetic trees were constructed to compare the individual Brassica species with A. thaliana RRA genes, as well as to compare all the Brassica RRA genes among themselves. Gene structure analysis of A. thaliana and Brassica RRA genes including their schematic representations was made using Gene Structure Display Server (http://gsds.gao-lab.org/) (Hu et al., 2015).
Dual synteny plots were created using the TBTools dual synteny plot function (Chen et al., 2020) to compare the Brassica species with A. thaliana, and B. napus with its parental species, B. rapa and B. oleracea. Before plotting the dual synteny, a one-step MCScanX analysis was performed in TBTools. The genome sequence files and gene structure annotation files for Brassica species and A. thaliana were retrieved from EnsemblPlants (Release 54) (Cunningham et al., 2021).
Plant materials, hormones, and abiotic stress treatment
Seeds of A. thaliana (Col-0), B. rapa (R-0-18), B. oleracea (DH1012), and B. napus (Darmor) were cultivated on 1/2 Murashige and Skoog (MS) medium for 1 week inside a growth chamber under controlled conditions. Before cultivation, the seeds underwent a cold pre-treatment in darkness at 4 °C for 3 d. The growth chamber was maintained at a temperature of 21 °C /18 °C for a 16 h day/8 h night photoperiod, with 130 µmol m–2 s–1 light intensity.
To investigate the expression profile of the 10 A. thaliana RRA and 66 Brassica RRA genes after cytokinin treatment, 1-week-old seedlings were exposed to exogenous treatment with 5 µM 6-benzylaminopurine (BAP) for 0, 0.5, 1, 2, and 4 as described by D’Agostino et al. (2000).
For the abiotic stress treatment, 1-week-old were incubated at 4 °C in the presence of white light for cold treatment. For salinity stress, the seedlings were treated with a 250 mM NaCl solution, and for osmotic stress, the seedlings were treated with a 300 mM mannitol solution. For the control treatment, the seedlings were treated with water only. Both the control and stress-treated seedlings were incubated in the growth chamber with a set temperature of 21 °C, with a light intensity of 130 µmol m–2 s–1 for 2 h and 4 h.
Additionally, a separate cold treatment experiment was conducted following the methodology described above to assess the expression of cold-responsive ARR7 and its Brassica homologs. The focus of this experiment was to evaluate the effects of the purine derivative PI-55, a known antagonist of cytokinin receptor activity (Spichal et al., 2009). One-week-old seedlings were treated with either PI-55 (0.1 µM and 1 µM) or 0.1% DMSO, and incubated under either cold (4 °C) or control conditions (21 °C) for 4 h.
RNA isolation and quantitative reverse transcription–PCR analysis
Total RNA was extracted from the collected seedlings following the Quick-Start Protocol included in the RNeasy® Plant Mini Kit (QIAGEN, Germany). Additionally, DNase treatment was performed using an RNase-Free DNase set (QIAGEN) to remove any DNA contamination. The concentration, integrity, and purity of the extracted RNA samples were examined using a NanoDrop One UV spectrophotometer (Thermo Fisher Scientific). Reverse transcription was performed to generate first-strand cDNA using the SuperScript™ III First-Strand Synthesis System (Thermo Fisher Scientific) with 1 µg of RNA using oligo(dT) primer. For the expression profiling of RRA genes after cytokinin treatment and abiotic stress exposure, 66 out of the 78 Brassica RRA genes along with the 10 A. thaliana RRA genes were analyzed. For the expression profiling of cold-responsive ARR genes after PI-55 treatment, ARR7 and its Brassica homologs (i.e. BrRRA7b, BoRRA7a, BoRRA7b, BnARRA7a, BnARRA7b, BnCRRA7a, and BnCRRA7b) were analyzed. Several reference genes were utilized as an internal control, including commonly used housekeeping genes (Guénin et al., 2009) such as UBQ10 and UBC10 (added for abiotic stress) for Arabidopsis, BrELF1 for B. rapa, BoTUB6 for B. oleracea, and BnACT2A and BnACT2C for B. napus (primers listed in Supplementary Table S3). All primers used were designed based on the following features: product size (70–200 bp), primer length (18–22 bp), Tm (59–65 °C), GC content (50–60%), target gene specificity, and absence of nucleotide repeats. Quantitative reverse transcription–PCRs were performed using FastStart SYBR® Green Master (Roche Diagnostics GmbH) on the Rotor-Gene Q 5plex HRM Platform (QIAGEN, Germany). Melting curve analysis was performed to confirm the specificity of the product for each primer pair. The relative gene expression level was calculated relative to the control using the delta-delta Ct method (Pfaffl, 2004). The RT–qPCR analysis was performed in three independent biological replicates, each with three technical replicates. Subsequently, a heatmap representation of the expression of RRA genes after exogenous cytokinin treatment and abiotic stress treatment was generated and is presented as the log2 fold change (log2FC). The heatmap was constructed using Cluster 3.0 for Windows (de Hoon et al., 2004) and viewed using Java TreeView (Saldanha, 2004).
Analysis of cis-regulatory elements in the promoter regions of RRA genes across Brassica species
Multiple sequence alignment of the homologous RRB amino acid sequences from Brassica species and A. thaliana was performed using Clustal Omega (Madeira et al., 2022) to assess the conservation of their GARP-like DNA-binding domains. The alignment was visualized using the MView online tool (Madeira et al., 2022). Reference genomes and genome annotations for A. thaliana, B. rapa, B. oleracea, and B. napus were downloaded from EnsemblPlants (Yates et al., 2022). The upstream regulatory sequences of protein-coding genes were extracted from the reference genomes using GFF3 annotations with the Bedtools getfasta tool (Quinlan and Hall, 2010). The publicly available ChIP-seq data for A. thaliana transcription factors (TFs) ARR1 and ARR10 (Xie et al., 2018) was used for a de novo motif search with Homer (Heinz et al., 2010). To identify potential RRB-binding sites in gene regulatory regions, Position Weight Matrices (PWMs) were used. The thresholds for PWMs were calculated using the previously described algorithm (Touzet and Varré, 2007). Then the PWMs were applied to three 500 bp long intervals of protein-coding genes: [–1500; –1000], [–1000; –500], and [–500; +1] relative to the transcription start site. To compare the density of potential RRB-binding sites in the regulatory regions of Brassica RRA-coding genes (used in the cytokinin and abiotic stress treatment) with random expectation (which is the density of the binding sites in the regulatory regions of all protein-coding genes), Fisher’s exact test was used. To account for multiple testing, we used Bonferroni correction: the P-value threshold was set as 0.05/24. The fold enrichment was calculated as the ratio of RRB-binding site density in RRA regulatory regions to the average density in the corresponding regions of all protein-coding genes.
The promoter sequences of A. thaliana and Brassica RRA genes (used in the cytokinin and abiotic stress treatment) were also subjected to in silico analysis using the online database, PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Lescot et al., 2002). The objective was to investigate the presence of environmental stress-responsive cis-elements in these sequences. Additionally, a Pearson correlation analysis was conducted to determine the relationship between the gene expression of cold-responsive A. thaliana RRA genes (ARR6, ARR7, and ARR15) and Brassica RRA genes (BrRRA6, BrRRA7a, BrRRA7b, BrRRA15a, BrRRA15b, BoRRA6, BoRRA7a, BoRRA7b, BoRRA15a, BoRRA15b, BnARRA6, BnARRA7a, BnARRA7b, BnARRA15a, BnCRRA6, BnCRRA7a, BnCRRA7b, BnCRRA15a, and BnCRRA15b) after 2 h and 4 h of cold exposure, and the total number of environmental stress-related cis-elements within the promoter regions of these genes. In the case of A. thaliana, additional comparisons were made using DAPseq data to select TFs with potential binding sites in the A. thaliana promoters. Moreover, to assess the enrichment of the TF-binding sites, particularly the PWM models in A. thaliana, a comparison was made between stress-sensitive promoters and stress-insensitive promoters for both A. thaliana and Brassica species.
Transformation of Brassica species with TCSv2:3×VENUS, cytokinin, and abiotic stress treatment
The TCSv2:3×VENUS construct, obtained from Maya Barr (Steiner et al., 2020), was subcloned into the pGREEN00279 binary vector (Hellens et al., 2000) and introduced into B. rapa (R-0-18), B. oleracea (DH1012), and B. napus (Darmor), following the protocol described by Jedlickova et al. (2022). Only root tips of B. rapa and B. napus transformed hairy roots were used in the experiment, as the transformation for B. oleracea was unsuccessful. Root tips of B. rapa and B. napus hairy roots were gathered 2 weeks after subculturing and treated with either 5 µM synthetic BAP or 0.1% DMSO for 0, 0.5, 1, 2, and 4 h, as described by D’Agostino et al. (2000), to test the cytokinin responsiveness of TCSv2:3×VENUS in the Brassica species. A total of three biological replicates were performed, with five roots for each replicate.
For stress treatments, the root tips of transformed B. rapa and B. napus hairy roots were exposed to 4 °C in the presence of white light for cold treatment. For salinity stress, the hairy roots were treated with 250 mM NaCl solution, and for osmotic stress they were treated with a 300 mM mannitol solution. For the control treatment, the hairy roots were treated with water only. Both the control and stress-treated hairy roots were incubated in the growth chamber with a set temperature of 21 °C, with a light intensity of 130 µmol–2 s–1 for 2 h and 4 h. A total of three biological replicates were performed, with 15 roots per replica.
Root imaging, and quantification of reporter gene expression
Root tips were imaged using the laser scanning confocal imaging microscope Zeiss LSM780 Axio-Observer, equipped with an external In Tune laser (488–649 nm, <3 nm width, pulsed at 40 MHz, 1.5 mW C-Apochromat) and a ×20 objective. The expression of VENUS in the root apical meristem (RAM) was quantified using IMAGEJ software (Schneider et al., 2012) and the spot detection algorithm in IMARIS 9.0 (Bitplane, http://www.bitplane.com/imaris/imaris). Representative images generated using IMARIS are presented. To ensure accurate analysis, the fluorescence intensity of each DMSO- or BAP-treated root was initially normalized to the area of the scanned roots (in pixels) and further normalized to the fluorescence intensity of the roots at the start of the treatment (0 h). Subsequently, the relative fluorescence intensity was calculated as the ratio of normalized fluorescence intensity in BAP-treated roots to the normalized fluorescence intensity of DMSO-treated roots.
Statistical analysis
A one-way ANOVA followed by Dunnett’s test was conducted to evaluate differences in the calculated relative fluorescence intensity in the scanned roots at the start and after 0.5, 1, 2, and 4 h of exogenous BAP treatment. Furthermore, a two-way ANOVA followed by Tukey’s HSD multiple comparison test was employed to compare the relative expression of cold-responsive ARR7, BrRRA7a, BrRRA7b, BoRRA7a, BoRRA7b, BnARRA7a, BnARRA7b, BnCRRA7a, and BnCRRA7b after PI-55 treatment. Statistical analysis was conducted using the GraphPad Prism version 9.0 for Windows (GraphPad Software, San Diego, CA, USA).
Results
The type-A response regulators and their genomic distribution in the Brassicaceae family
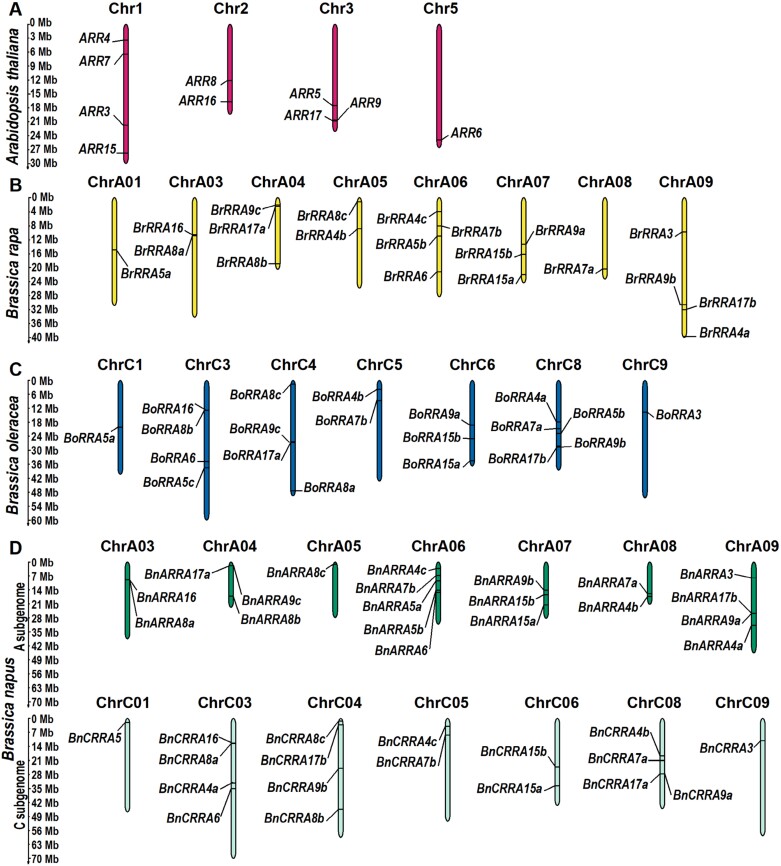
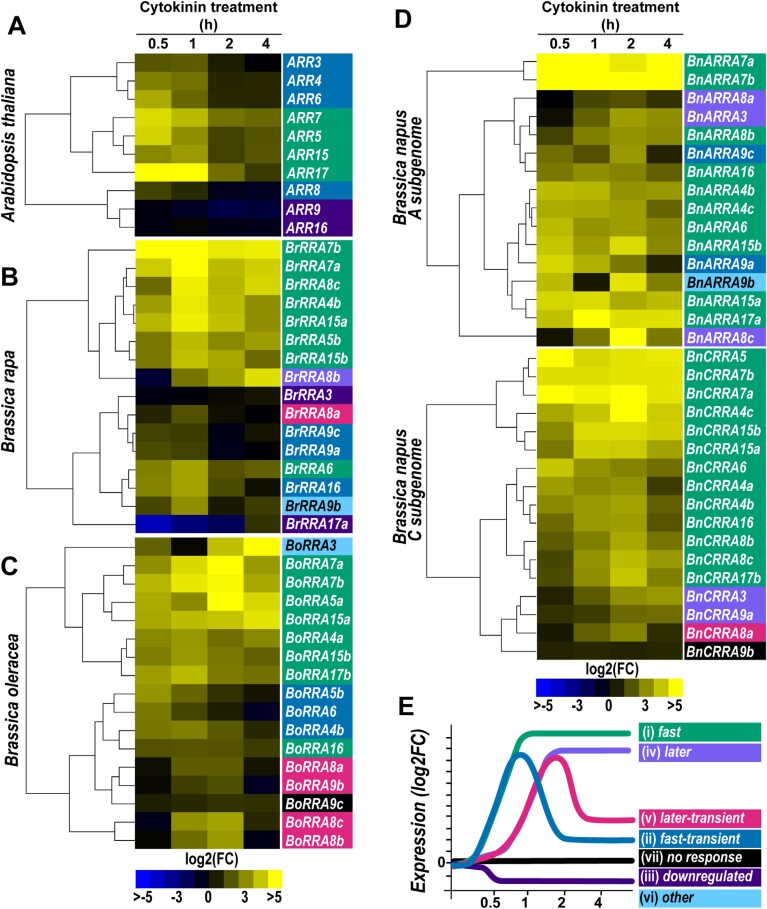
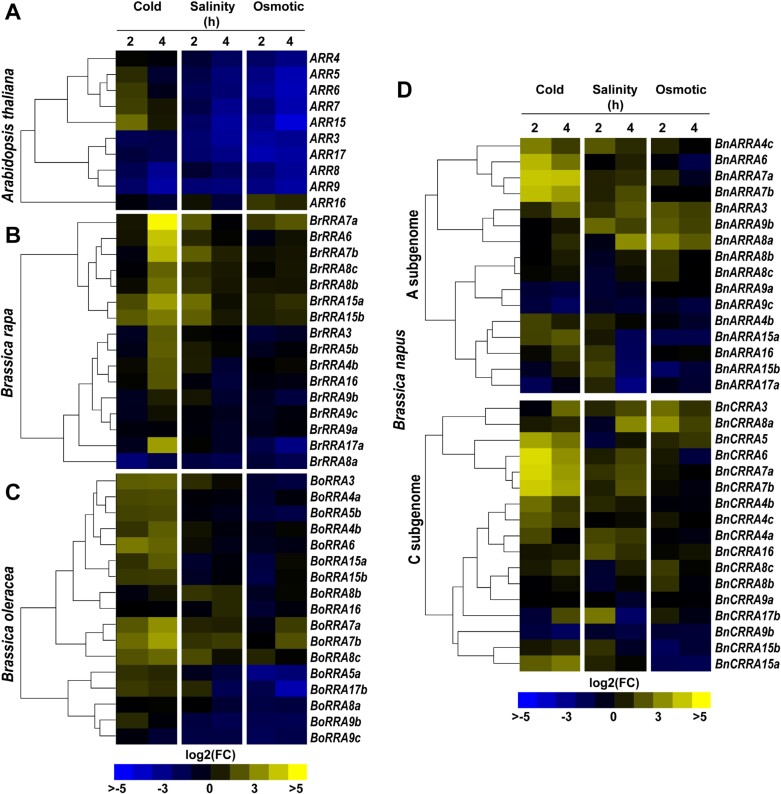
Using a similarity search (see the Materials and methods for more details), we identified 78 putative RRA genes in B. oleracea, B. rapa, and B. napus that share a high degree of sequence identity with A. thaliana RRA genes (Fig. 1A–D; Supplementary Table S2). Among these, 20 and 15 were previously reported in the genome of B. rapa and B. oleracea, respectively (Liu et al., 2014; Kaltenegger et al., 2018), thus affirming the robustness of our bioinformatic search methodology. Following previously agreed nomenclature (Heyl et al., 2013), we designated them as BrRRA and BoRRA genes (Fig. 1B, C). In the genome of B. oleracea, we found five novel putative RRA genes that were not included in Kaltenegger et al. (2018) (Fig. 1C). In B. napus, we recognized 38 novel putative RRA genes, 20 of which located in the A subgenome (BnARRA genes) and 18 in the C subgenome (BnCRRA genes) (Fig. 1D). The putative paralogs were indexed with ‘a’, ‘b’, or ‘c’ in an order following the decreasing percentage of amino acid identities they share with the corresponding A. thaliana RRA (ARR; Fig. 1A).
Fig. 1.
Chromosomal localization of known and newly identified RRA genes in Brassicaceae. RRA genes in (A) Arabidopsis thaliana, (B) Brassica rapa, (C) Brassica oleracea, and (D) A and C subgenome of Brassica napus. Each panel displays only the chromosomes (designated as ‘Chr’) where the RRA genes were identified.
BrRRA genes were mapped to chromosomes ChrA01, ChrA03, ChrA04, ChrA05, ChrA06, ChrA07, ChrA08, and ChrA09, while BoRRA genes were located on ChrC1, ChrC3, ChrC4, ChrC5, ChrC6, ChrC8, and ChrC9 (Fig. 1B, C). As expected, BnARRA and BnCRRA genes were found on corresponding homologous chromosomes in the A and C subgenomes, respectively (ChrA03, ChrA04, ChrA05, ChrA06, ChrA07, ChrA08, and ChrA09 for BnARRA genes, and ChrC01, ChrC03, ChrC04, ChrC05, ChrC06, ChrC08, and ChrC09 for BnCRRA genes; Fig. 1D).
Brassica and A. thaliana RRAs show a high level of conservation
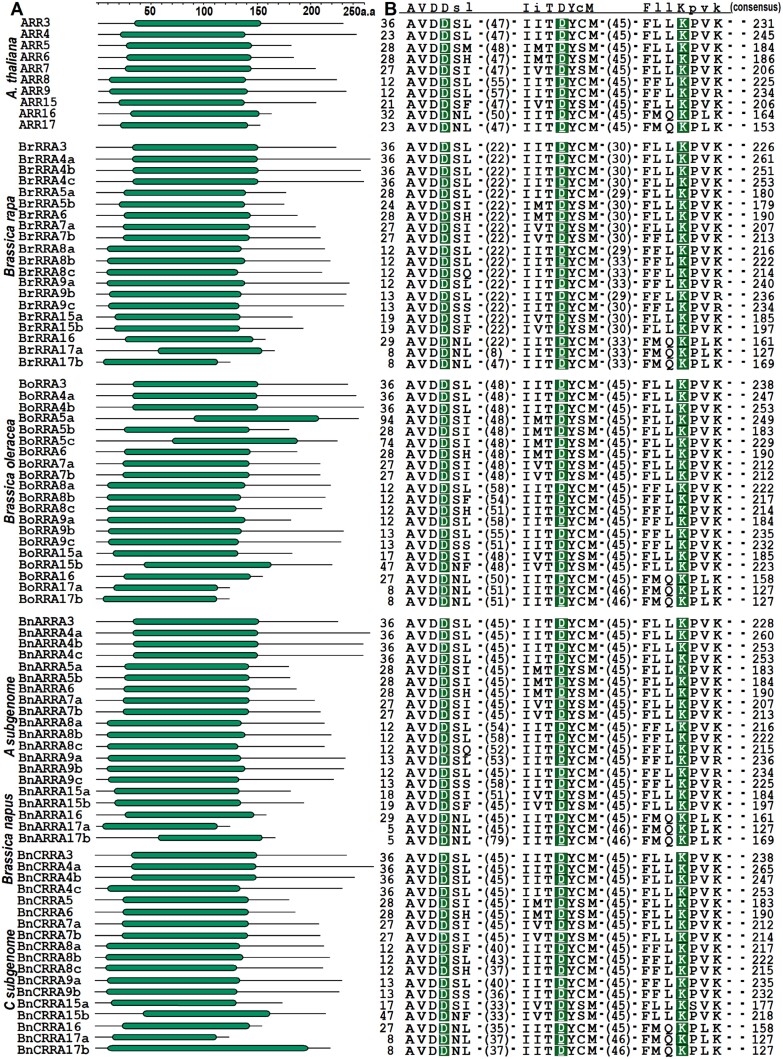
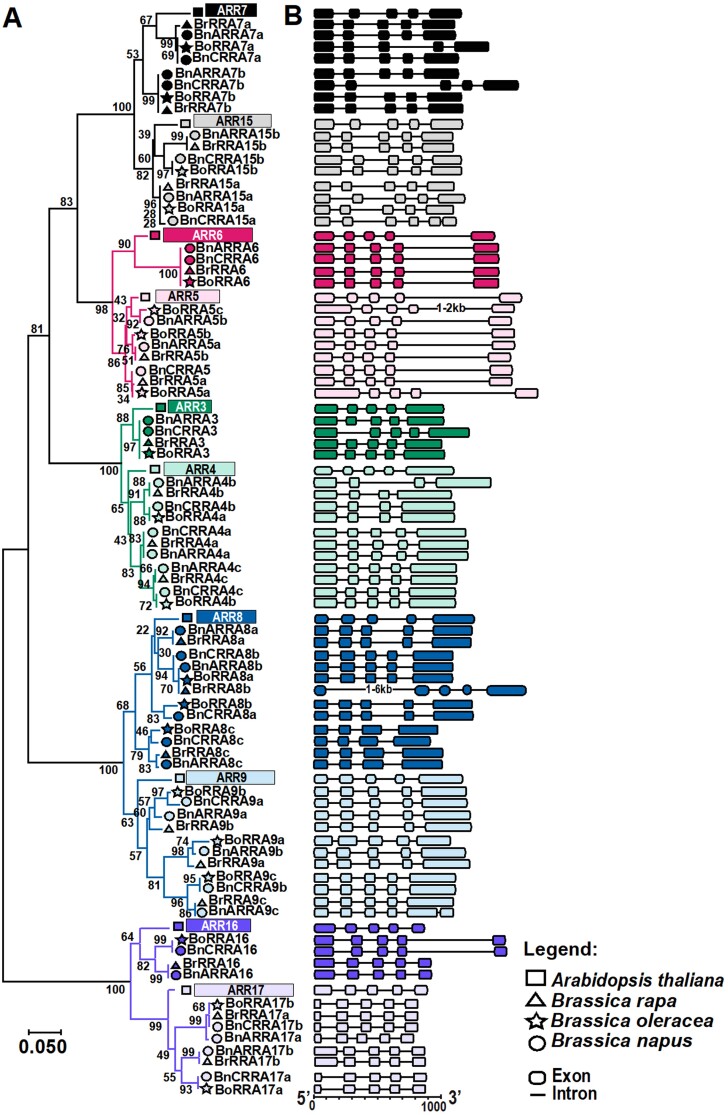
A motif search in the putative protein sequences of all the 78 Brassica RRAs confirmed the presence of the conserved Rec domain harboring the highly conserved D-D-K motif, including the (underlined) phosphoaccepting Asp, which is essential for the role of RRAs in mediating the negative feedback regulation of cytokinin signaling (Lee et al., 2008) (Fig. 2A, B). Moreover, all the predicted Brassica RRAs had protein sizes comparable with their putative A. thaliana orthologs (identified based on their phylogenetic analysis, see later in the text and Fig. 3), ranging from 127 to 265 amino acid residues, with ARR4 and ARR17 and their homologs being the longest and shortest, respectively (Fig. 2; Supplementary Table S2). The evolutionary relationship among the RRAs (78 Brassica and 10 A. thaliana RRAs) was assayed by aligning the amino acid sequences of conserved Rec domains (Fig. 3A; Supplementary Figs S1–S3). As expected, we observed a high level of conservation between the RRAs from Brassica sp. and A. thaliana. The tree consists of five main clades, each composed of two subclades, reflecting the presence of five couples of very similar/paralogous RRAs (ARR7/ARR15, ARR5/ARR6, ARR3/ARR4, ARR16/ARR17, and ARR8/ARR9). This information was used to designate the individual Brassica RRAs according to their clustering into individual paralogous subclades (Fig. 3A).
Fig. 2.
Arabidopsis thaliana and Brassica RRA genes reveal a high level of domain structure and amino acid sequence conservation. (A) Schematic depiction of the protein domain of RRAs from A. thaliana and Brassica sp., showing the localization of the receiver domain (Rec, as a green rounded rectangle, and the rest of the amino acid residue as lines). The top line shows the amino acid residue (a.a) position coordinates. (B) Multiple sequence alignment of several amino acid sequences adjacent to the conserved D-D-K motif (green box) in the Rec domain of the individual RRA protein sequences. The numbers of amino acid residues preceding the residues shown in the figure (numbers at the start and in the middle of RRA protein sequences), along with the total number of amino acid residues for each RRA protein sequence (number at the end), are indicated. The consensus sequence is displayed above the alignment; conserved residues are in uppercase, while lowercase characters represent the most common amino acids at variable positions.
Fig. 3.
Phylogenetic relationships and gene structures of RRA genes in Brassicaceae. (A) The unrooted tree is based on the similarity of RRA Rec domains constructed using the Neighbor–Joining method; the bar represents the relative divergence of the examined sequences. The subclades composed of RRAs potentially orthologous to individual A. thaliana RRA genes are presented using the same color; the subclades comprising homologs of the paired A. thaliana RRA genes, the result of an α WGD event (see the main text for details), are distinguished by different shades of a given color. The RRAs from individual species are distinguished by a triangle (BrRRAs), star (BoRRAS), and circle (BnRRAs). (B) A schematic representation of the A. thaliana and Brassica RRA gene structures (exons are depicted as boxes separated by introns as lines); the color code is used as in (A).
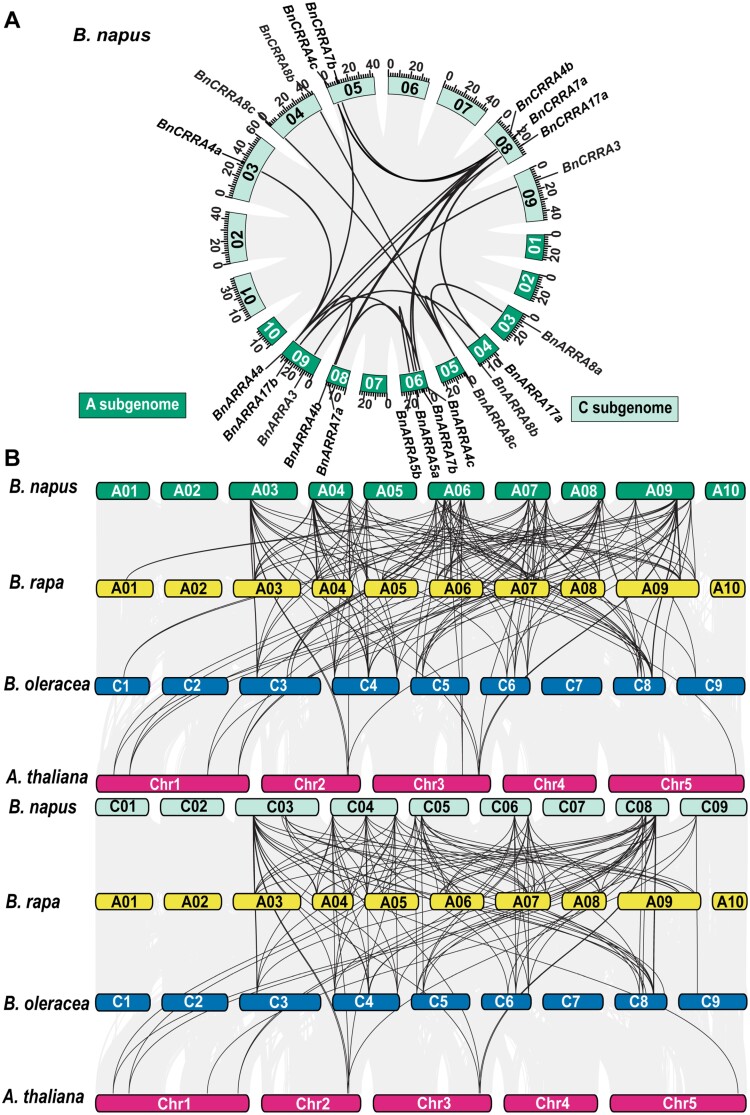
The analysis of gene structure revealed that, except for eight RRA genes containing only four exons (BrRRA4b, BoRRA4a, BnARRA4b, BnCRRA4b, BoRRA8c, BrRRA8c, BnCRRA8c, and BnARRA8c), all other RRA genes shared a gene model consisting of five exons and four introns (Fig. 3B). Among these, ARR6 and its Brassica homologs exhibited nearly identical gene structures, including the number and length of exons and introns. Furthermore, genome-to-genome synteny analysis between the individual Brassica species and A. thaliana (Fig. 4A) revealed that 20 out of 20 BrRRA genes, 11 out of 20 BoRRA genes, and 32 out of 38 BnRRA genes genes were syntenic with their A. thaliana counterparts. In the case of B. napus, 36 out of 38 BnRRA genes were syntenic with those of B. rapa and B. oleracea. Within B. napus subgenomes, 18 paralogous gene pairs displayed segmental duplications. Among these, nine pairs were segmental duplications between the six BnARRA and seven BnCRRA genes, two pairs were segmental duplications involving four BnCRRA genes, and seven pairs were segmental duplications between 10 BnARRA genes (Fig. 4A).
Fig. 4.
The syntenic conservation of B. napus RRA genes. (A) Synteny of the BnRRA genes. Gray lines represent syntenic blocks in the B. napus genome, while black lines indicate paralogous BnRRA gene pairs, demonstrating segmental duplication between different chromosomes. The A and C subgenomes are distinguished by the color difference in the box bearing the chromosome name. The scale at the bottom of these boxes represents the size of the chromosome in megabases. (B) Collinearity of B. napus (A and C subgenome), B. rapa, B. oleracea, and A. thaliana genomes. Gray lines illustrate collinear blocks among these species, while black lines show the orthology in the BnRRA, BrRRA, BoRRA, and A. thaliana RR genes. The dark and light green boxes represent the chromosomes in the A and C subgenomes of B. napus, the yellow boxes for the B. rapa chromosomes, the blue boxes for the B. oleracea chromosomes, and the dark pink boxes for the A. thaliana chromosomes (designated as ‘Chr’).
Taken together, a high level of amino acid sequence conservation was observed within the Brassica species, confirming the previously described evolutionary relationships (Morinaga, 1929; Nagaharu and Nagaharu, 1935; Cheng et al., 2012, 2014; Nikolov et al., 2019; Hendriks et al., 2023).
Cytokinin treatment revealed the shared and distinct patterns of the RRA expression profiles between A. thaliana and Brassica sp.
The A. thaliana RRA genes are considered primary cytokinin response genes, as their transcription is promptly induced by exogenous cytokinins even in the absence of de novo protein synthesis (Taniguchi et al., 1998; D’Agostino et al., 2000). To compare the effects of cytokinin on the expression of A. thaliana and Brassica RRA genes, 1-week-old A. thaliana and Brassica seedlings were exposed to exogenous cytokinins for various times ranging from 30 min to 4 h (Fig. 5; Supplementary Table S4).
Fig. 5.
Kinetics of A. thaliana and Brassica RRA gene response to cytokinins. Heatmaps represent the relative change of RRA expression in the 1-week-old seedlings after cytokinin (5 µM BAP) treatment for the given time (0.5, 1, 2, and 4 h) normalized to mock-treated controls in (A) A. thaliana, (B) B. rapa, (C) B. oleracea, and (D) B. napus. The expression data are presented as log2 fold change between BAP- and mock-treated samples normalized by the delta-delta Ct (Pfaffl, 2004). (E) Schematic depiction of identified expression profile categories. The categorization of individual RRAs in (A–D) is color-coded as defined in (E).
Based on the time course of the observed transcriptional response, the expression profiles of individual A. thaliana RRA genes were classified into three categories: (i) fast, exhibiting prompt up-regulation after 30 min of cytokinin treatment followed by a gradual decline of expression throughout the rest of the treatment period; (ii) fast-transient, similar to (i), but revealing a fast decline after the initial peak; and (iii) down-regulated, indicating a reduced expression throughout the experiment (Fig. 5A, E; Supplementary Table S4). In A. thaliana, we observed the same number (four) of RRA genes with cytokinin response profiles classified as fast and fast-transient and two RRA genes belonging to the down-regulated category (Fig. 5A). In contrast, in B. rapa and B. oleracea, the proportion of RRA genes with the fast profile increased at the expense of the fast-transient. Additionally, four additional categories emerged: (iv) later, characterized by delayed up-regulation occurring after 1 h of cytokinin treatment and persisting until 4 h; (v) later-transient, similar to the later category but with a decline in expression at 4 h; (vi) other, showing various response types; and (vii) no response (Fig. 5B–D). The decrease in the number of RRA genes of the fast-transient category was more pronounced in B. oleracea compared with B. rapa. This trend was even more evident when comparing the A and C subgenome-specific RRA genes in B. napus, where at least two RRA genes of the fast-transient profile were still retained among the BnARRA genes (encoded by the A subgenome of B. rapa origin), but no fast-transient RRA profile was found among BnCRRA genes (located in the C subgenome originating from B. oleracea; compare Fig. 5B–D).
Analyzing the cytokinin response of individual RRA genes across the Brassica species and A. thaliana, similar expression profiles were observed for ARR5, ARR7, and ARR15, and most of their homologs in B. rapa, B. oleracea, and B. napus. However, a higher level of expression change (log2FC) of these RRA genes was observed in the Brassica species compared with A. thaliana, and this trend was apparent in particular for B. napus homologs of ARR7 (Fig. 5; Supplementary Table S4). This aligns with RNA-sequencing profiling results of B. napus cultivars using the Renewable Industrial Products from Rapeseed (RIPR) diversity panel (Havlickova et al., 2018), which identified ARR7 orthologs as one of the most abundant RRA genes among the B. napus cultivars (Supplementary Fig. S4).
To sum up, all assayed RRA genes across the Brassicaceae family were up-regulated by cytokinins, demonstrating partially overlapping, but also species-specific temporal expression patterns.
Cytokinin-induced up-regulation of Brassica RRA genes via motifs recognized by RRBs is conserved in Brassicaceae
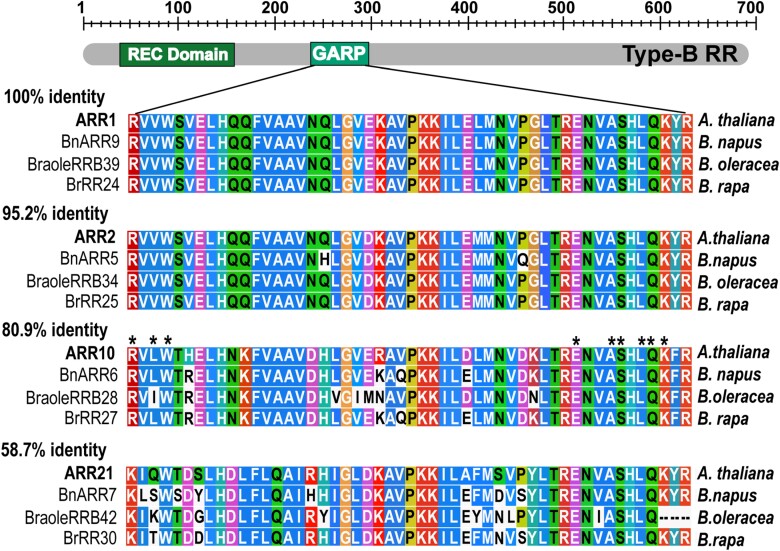
In Arabidopsis, cytokinin-dependent transcriptional activation of RRA genes is mediated by RRBs, the cytokinin-regulated TFs that bind specific cis-regulatory motifs enriched in the promoters of cytokinin-responsive genes (Muller and Sheen, 2008). To assess the possible conservation of DNA targets recognized by RRBs in A. thaliana and Brassica species, we performed a multiple protein sequence alignment of DNA-binding GARP-like domain of A. thaliana RRBs ARR1, ARR2, ARR10, ARR11, ARR12, ARR13, ARR18, ARR19, ARR20, and ARR21 (Sakai et al., 2000; Lohrmann et al., 2001; Hosoda et al., 2002; Mason et al., 2005) and their putative orthologs previously identified in the Brassica sp. (Liu et al., 2014; Kaltenegger et al., 2018; Jiang et al., 2022). A high level of conservation was observed, with the identity in amino acid sequence ranging from 100% for ARR1, 95.2% for ARR2, and 80.9% for ARR10, to 58.7% in the case of ARR21 (Fig. 6; Supplementary Fig. S5). Given this high conservation of the GARP-like DNA-binding domain across the A. thaliana and Brassica RRBs, it is likely that the Brassica RRBs recognize DNA-binding motifs similar to those previously described in A. thaliana (Sakai et al., 2000; Hosoda et al., 2002; Imamura et al., 2003; Zubo et al., 2017; Xie et al., 2018).
Fig. 6.
The DNA-binding domain of A. thaliana and Brassica RRBs shows a high level of amino acid conservation. Domain structure of A. thaliana and Brassica RRBs and alignment of the amino acid sequences of the GARP-like DNA-binding domain for the selected RRBs from A. thaliana and assayed Brassica species. Conserved amino acids are highlighted, and the percentage identity is shown. The CLUSTAL color scheme was used to color the alignment, reflecting the physicochemical properties of amino acids (Kunzmann et al., 2020). The asterisk denotes the ARR10 residues proposed to interact directly with DNA (Hosoda et al., 2002); for a comprehensive list of RRB alignments, refer to Supplementary Fig. S5.
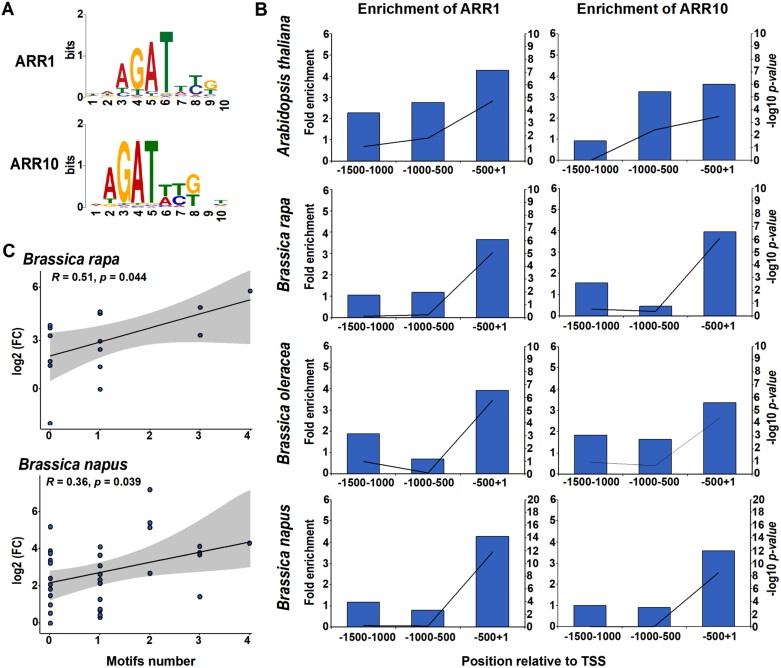
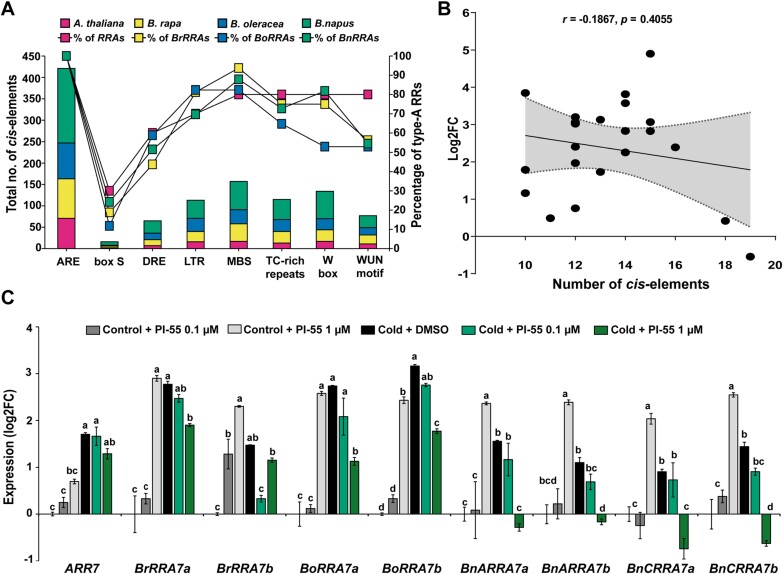
To further corroborate this assumption, we utilized the PWMs for the A. thaliana ARR1 and ARR10 DNA-binding sites, retrieved from the ChIP-seq peak sets (Zubo et al., 2017; Xie et al., 2018) to predict putative RRB-binding sites within the Brassica species (Fig. 7A). Using this approach, the presence of Arabidopsis-like cytokinin-responsive cis-elements was predicted in the [–1500; +1 relative to the transcription start site] regulatory regions of 62 out of the 66 analyzed Brassica RRA genes used in the cytokinin treatment (Supplementary Table S5). Similar to A. thaliana, these potential cis-elements were significantly enriched within the proximal 5'-regulatory regions of Brassica RRA genes (within 500 bp upstream of the transcription start site; Fig. 7B). We also observed a moderate correlation between the number of motifs within the [–500; +1] regulatory regions and the magnitude of the transcriptional response to cytokinin, which was statistically significant in B. napus and B. rapa (Fig. 7C). This finding further supports the notion of the functional role of Arabidopsis-like cis-elements in regulating the transcriptional response to cytokinins in the assayed Brassica species and suggests a possible role for motif clustering in the response amplification.
Fig. 7.
Promoters of Brassica RRA genes are enriched for the Arabidopsis-like cytokinin-responsive cis-regulatory elements. (A) The Position Weight Matrix (PWM) for the ARR1 and ARR10 DNA-binding sites in A. thaliana was retrieved from ChIP-seq peak sets (Xie et al., 2018). (B) Significant enrichment of ARR1 and ARR10 PWM hits proximal to 5'-regulatory regions of A. thaliana and Brassica RRA genes. Bars represent fold enrichment (left axis) and the line represents –log10 P-value (right axis). (C) Significant correlation (Pearson correlation with 95% confidence intervals, shadowed part) between the transcriptional response to cytokinin of BrRRA and BnRRA genes and the number of cytokinin-responsive motifs present in their promoter regions.
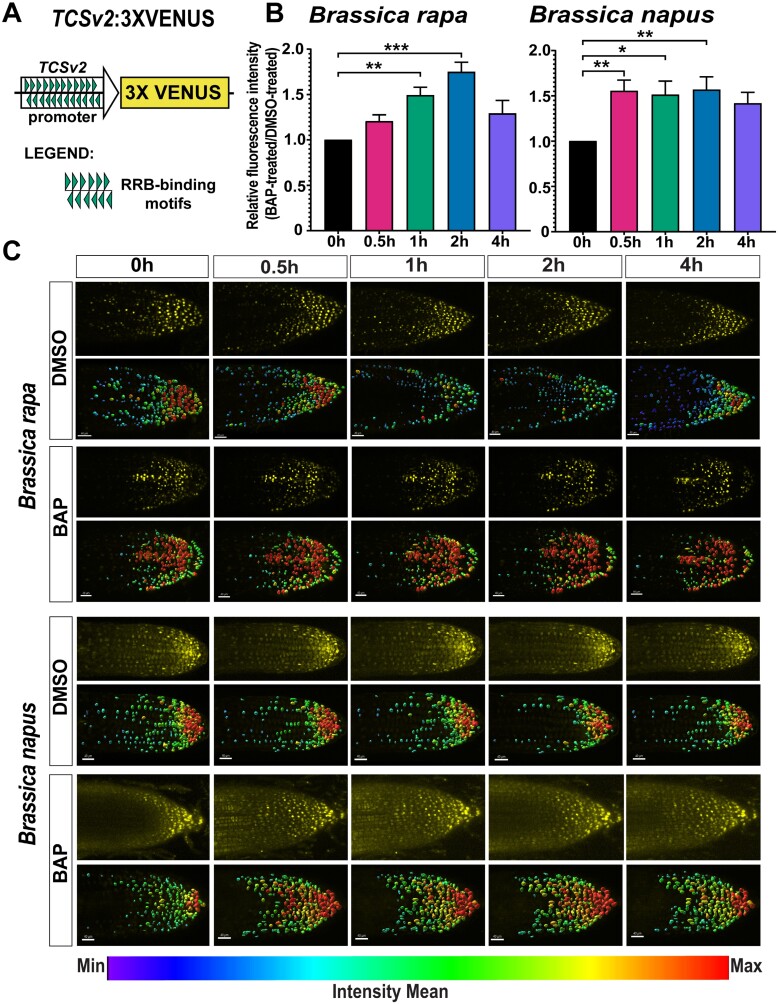
To validate these findings, we utilized the hairy root transformation system (Jedlickova et al., 2022) to introduce the cytokinin-responsive reporter (TCSv2:3×VENUS) developed in A. thaliana by Steiner et al. (2020) into Brassica species. TCSv2 incorporates concatemerized RRB-binding motifs with a distinct arrangement (Fig. 8A) that enhances sensitivity when compared with the original version of the TCS reporter (Zurcher et al., 2013). Compared with a mock-treated control, a significant increase in the relative fluorescence intensity was observed after 30 min and 1 h of the cytokinin treatment in the hairy roots of B. napus and B. rapa, respectively, carrying TCSv2:3×VENUS (Fig. 8B, C).
Fig. 8.
The Arabidopsis TCSv2:3×VENUS cytokinin reporter (Steiner et al., 2020) is cytokinin responsive in B. rapa and B. napus. (A) Scheme of the TCSv2:3×VENUS (after Steiner et al., 2020). (B) Comparison of the relative fluorescence intensity of the TCSv2:3×VENUS cytokinin reporter in BAP-treated hairy roots of B. rapa and B. napus at different time points (0.5, 1, 2, and 4 h) of cytokinin (5 µM BAP) treatment. Means ±SE are shown in the plots. Asterisks indicate statistical significance (***P<0.001, **P<0.01, and *P<0.05, Dunnett’s test). (C) Representative images of B. rapa and B. napus hairy root tips treated with DMSO and BAP throughout the treatment period, showing the measured fluorescent signal intensities in a single root (top) and the corresponding image analyzed by IMARIS software (below). Scale bars represent 40 µm.
Taken together, our results strongly suggest that similarly to Arabidopsis, the Brassica RRBs recognize conserved cis-regulatory regions to mediate the cytokinin-induced transcriptional activation of Brassica RRA genes and possibly other cytokinin-responsive genes within the Brassica genomes.
Cold stress stimulates RRA expression in the Brassicaceae family
To assay the possible stress-related regulation of RRA genes within the Brassicaceae family, the expression profiles of the 66 selected Brassica RRA genes and the 10 A. thaliana RRA genes were investigated after exposure to cold (4 °C), salinity (250 mM NaCl), and osmotic stress (300 mM mannitol). In A. thaliana, cold stress rapidly (within 2 h after the stress application) up-regulated the expression of several RRA genes, in particular ARR6, ARR7, and ARR15. However, the cold-induced up-regulation was transient, and the expression of up-regulated RRA genes returned to basal levels after 4 h of cold exposure. In contrast, we observed gradual repression of ARR3, ARR8, ARR9, ARR16, and ARR17 at 2 h and 4 h of the cold stress application (Fig. 9A; Supplementary Table S6). In B. rapa, greater numbers of RRA genes were up-regulated in the response to cold, although the induction was delayed when compared with A. thaliana. Most BrRRA genes, except for the non-responsive BrRRA8a, BrRRA9a, BrRRA9b, and BrRRA9c, exhibited up-regulation after 4 h of cold exposure. BrRRA15a and BrRRA15b showed an earlier response, being up-regulated after 2 h of chilling, and remained activated for the 4 h of the treatment (Fig. 9B; Supplementary Table S6). Also in B. oleracea, most of the BoRRA genes were up-regulated by cold stress. Similarly to A. thaliana, the response was evident early (2 h) during cold exposure; however, compared with the transient up-regulation seen in the cold-responsive A. thaliana RRA genes, the up-regulation of BoRRA genes lasted the entire 4 h of treatment. This response pattern was observed for BoRRA6, BoRRA7a, BoRRA7b, BoRRA15a, and BoRRA15b (Fig. 9C; Supplementary Table S6). Also in B. napus, we observed prompt up-regulation of RRA genes lasting for the 4 h of the cold treatment. This type of response was apparent for homologs of ARR6 (BnARRA6 and BnCRRA6), ARR7 (BnARRA7a, BnARRA7b, BnCRRA7a, and BnCRRA7b), and ARR15 (BnARRA15a and BnCRRA15b). Several other BnRRA genes, including homologs of ARR3, ARR4, ARR5, ARR8, and ARR17, were also up-regulated by cold, but with variable kinetics (Fig. 9D; Supplementary Table S6).
Fig. 9.
Arabidopsis thaliana and Brassica RRA genes respond to abiotic stress. Heat maps depicting the expression pattern of RRA genes in 1-week-old seedlings of (A) A. thaliana, (B) B. rapa, (C) B. oleracea, and (D) B. napus under cold (4 °C), salinity (250 mM NaCl), and osmotic stress (300 mM mannitol) conditions for 2 h and 4 h (see the Materials and methods). The expression data are presented as log2 fold change normalized to the mock treatment by the delta-delta Ct (Pfaffl, 2004); for the color code see the key.
In summary, several RRA genes are up-regulated in response to cold stress in the Brassicaceae family, albeit with slightly different kinetics. ARR6, ARR7, ARR15, and their Brassica homologs appear to represent the core of the common cold-responsive transcriptional signature among the RRA genes.
Salinity and osmotic stress lead to contrasting expression of A. thaliana and Brassica RRA genes
Compared with cytokinin and cold treatment, the majority of A. thaliana RRA genes exhibited down-regulation after exposure to salinity and osmotic stress, except for ARR16, which showed up-regulation after 2 h of salinity stress (Fig. 9A; Supplementary Table S6). In contrast, several BrRRA genes were up-regulated after 2 h of salinity exposure, particularly the homologs of ARR6 (BrRRA6), ARR7 (BrRRA7a, 7b), and ARR15 (BrRRA15a and BrRRA15b). However, only BrRRA7b displayed up-regulation when exposed to osmotic stress (Fig. 9B; Supplementary Table S6). In B. oleracea, homologs of ARR7 (BoRRA7b and BoRRA7c) along with BoRRA8b and BoRRA8c were up-regulated after 2 h of salinity treatment, and this effect persisted up to 4 h, except for BoRRA8b. In response to osmotic stress, only homologs of ARR7 (BoRRA7a and BoRRA7b) were up-regulated after 4 h of treatment (Fig. 9C; Supplementary Table S6). In contrast to their diploid ancestors, there were more RRA genes in B. napus that were induced by salinity and/or osmotic stress after either 2 h or 4 h of stress exposure. These included BnARRA3, BnARRA7a, BnARRA7b, BnARRA8a, BnARRA8b, BnARRA8c, and BnARRA9b in the A genome and all RRA genes from the C-genome, except BnARRA9a and BnARRA9b.
Overall, RRA genes in Brassicaceae are regulated by salt and osmolarity stresses, displaying various types (up- versus down-regulation) and dynamics of the response. Compared with A. thaliana RRA genes being mostly down-regulated, all tested Brassica crops exhibited up-regulation of RRA genes in the presence of not only cytokinins but also of abiotic stresses. Similar to the cold treatment, homologs of ARR7 and ARR15 appear to be a sensitive readout of the response to salinity and high osmolarity in both diploid Brassica species, B. rapa and B. oleracea. However, particularly in B. napus, the response to these stress types seems to be more general, involving a larger number of RRA genes.
Cytokinin-responsive reporter TCSv2 as a sensitive tool for studying early stress responses
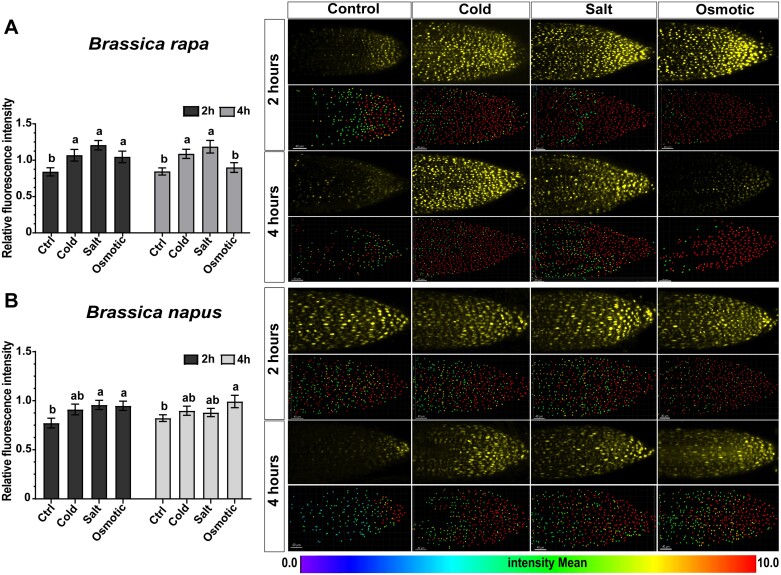
To elucidate the molecular mechanism of stress-induced RRA up-regulation in Brassica sp., we investigated the response of the TCSv2::3×VENUS reporter to various abiotic stresses in B. rapa and B. napus root tips (Fig. 10). Notably, under cold, salt, and osmotic stress conditions, in B. rapa TCSv2-driven VENUS exhibited a significant increase of intensity 2 h and 4 h post-treatment, with the exception of osmotic stress, showing significant up-regulation only after 2 h of the treatment (Fig. 10A). Compared with that, B. napus showed an induction of VENUS intensity by all stress treatments after 2 h, with salt and osmotic stress showing significant differences compared with the control. However, no significant differences were observed between treatments and control conditions at 4 h of treatment, except for osmotic stress (Fig. 10B). In conclusion, our observations clearly show sensitivity of the (RRB-regulated) cytokinin reporter system in Brassica species to abiotic stress, emphasizing its utility in discerning early stress responses in crops.
Fig. 10.
The Arabidopsis TCSv2:3×VENUS cytokinin reporter (Steiner et al., 2020) in B. rapa and B. napus is sensitive to early stress response. Comparison of the relative fluorescence intensity of the TCSv2:3×VENUS cytokinin reporter in control and abiotic stress-treated [cold (4 °C), salinity (250 mM NaCl), and osmotic stress (300 mM mannitol)] hairy roots of B. rapa (A) and B. napus (B) at different time points (2 h and 4 h; see the Materials and methods). Means ±SE are shown in the plots. The different letters indicate variable groups with statistically significant differences (P<0.05, Tukey’s HSD). Representative images of B. rapa (A) and B. napus (B) hairy root tips under control and abiotic stress throughout the treatment period, showing the measured fluorescent signal intensities in a single root (top) and the corresponding image analyzed by IMARIS software (below). Scale bars represent 40 µm.
Cytokinins contribute to the cold stress-induced up-regulation of RRA genes in Brassicaceae
Our gene expression data show the regulation of RRA genes by abiotic stresses. Utilizing the online database and PlantCARE portal (Lescot et al., 2002), several environmental stress-related cis-elements were identified in all the promoter sequences of A. thaliana RRA genes, 16 BrRRA genes and BnARRA genes, and 17 BoRRA genes and BnCRRA genes (Fig. 11A; Supplementary Table S7). However, the correlation tests between the number of identified stress-related cis-elements and the expression of cold-responsive ARR6, ARR7, ARR15, and their Brassica homologs after cold exposure did not yield any statistically significant results (Fig. 11B; Supplementary Fig. S6). In an alternative approach, we searched the DAP-seq data (Bartlett et al., 2017) to find TFs with potential binding sites in A. thaliana RRA promoters. We found six such TFs (AT2G28810, AT3G52440, AT5G56840, ATHB25, ATHB23, and ATHB34); however, the significance of enrichment of their binding sites in the stress-responsive A. thaliana and Brassica RRA genes was low (Supplementary Tables S8, S9). Altogether, our data do not provide any solid evidence supporting the role of the identified stress-related cis-regulatory elements in the control of RRA gene expression within the Brassicaceae family.
Fig. 11.
Role of cytokinins dominates over environmental stress-related cis-elements in cold-induced RRA up-regulation in Brassica species. (A) Comparison of the number of environmental stress-related cis-elements identified using the PlantCARE databases (Lescot et al., 2002) in the promoter regions of A. thaliana and Brassica RRA genes along with the percentage of RRA genes where these cis-elements were found. (B) Pearson correlation (with 95% confidence interval, shadowed part) between the transcriptional response of cold-responsive A. thaliana and Brassica RRA genes after 4 h of cold treatment and the number of environmental cis-elements present in their promoter regions. (C) Expression of ARR7 and its homologs after incubation of 1-week-old seedlings in medium supplemented with either DMSO or the cytokinin antagonist PI-55 (0.1 µM/1 µM) and exposure for 4 h to either cold or control conditions. The expression data are presented as log2 fold change double normalized by the delta-delta Ct (Pfaffl, 2004) (means ± SE) to the corresponding housekeeping gene (see the Materials and methods) and the control. The different letters indicate variable groups with statistically significant differences (P<0.05, Tukey’s HSD).
To assess the possible involvement of cytokinins in the cold stress-mediated up-regulation of RRA genes, we tested the cold response of ARR7 and its Brassica homologs in the presence of the anticytokinin (cytokinin signaling inhibitor) PI-55. PI-55 was demonstrated to inhibit the activation of the MSP signaling cascade by competing with cytokinin binding to the CHASE domain of AHKs (Spichal et al., 2009). Under control conditions, treatment with PI-55 led to the induction of all tested RRA genes, probably due to its previously reported weak cytokinin activity (Spichal et al., 2009). However, when applied under low-temperature conditions, PI-55 was able to reduce the cold-induced up-regulation of ARR7 and its Brassica homologs. However, it should be pointed out here that although a similar trend was apparent in all species tested (except for BrRRA7b), the effect was statistically significant only in B. oleracea and was particularly strong in B. napus, where the presence of 1 µM PI-55 completely abolished the up-regulation of cold-induced B. napus ARR7 homologs and led to the drop of gene expression even under the control levels (Fig. 10C).
In conclusion, our findings suggest the existence of a cytokinin-dependent mechanism that contributes to the activation of several RRA genes in the response to cold stress.
Discussion
Brassica and A. thaliana RRA genes reveal a close evolutionary relationship
We unearthed a total of 78 putative RRAs within the genomes of B. oleracea, B. rapa, and B. napus. Our investigation not only validates the prior identification of certain RRAs reported in the genomes of B. rapa and B. oleracea but also introduces novel candidates, expanding our understanding of the MSP regulatory landscape in these Brassica species from an evolutionary perspective.
Three rounds of whole-genome duplications (WGDs) took place in Brassicaceae after its lineage diverged from monocots but prior to the further divergence within the family (Moghe et al., 2014). Kalteneger et al. (2018) proposed the presence of two RRA copies (possibly resulting from the ancient ζ or ε WGD event) in the last common ancestor before the divergence of monocots and dicots. Four of the five paralogous RRA pairs (ARR6/ARR5, ARR15/ARR7, ARR8/ARR9, and ARR17/ARR16) (Kaltenegger et al., 2018) probably originated through the later α WGD event dated to ~47 million years ago (Mya). More recently (~25 Mya), an α' whole-genome triplication (WGT) event took place in the Brassica ancestor after the divergence from the Arabidopsis lineage (Lysak et al., 2005; Town et al., 2006; Yang et al., 2006; Wang et al., 2011), leading to the formation of 20 RRA genes in both B. oleracea and B. rapa.
The allotetraploid B. napus is a result of interspecific hybridization between B. rapa and B. oleracea (Nagaharu and Nagaharu, 1935; Zhang et al., 2016). In accordance with that, the 20 BnARRA genes identified in the A subgenome and 18 BnCRRA genes found in the C subgenome exhibit notable similarity and are mostly syntenic with their counterparts in the B. rapa and B. oleracea genomes, respectively. Considering the close evolutionary relationships, we used the well-established A. thaliana RRA genes (ARR genes) as a reference and numbered the newly identified B. napus RRA gene according to their (putative) A. thaliana orthologs. For the sake of consistency, we extended this type of numbering to the newly identified BoRRA genes as well as to the previously described BrRRA and BoRRA genes (Kaltenegger et al., 2018). We believe that this nomenclature type facilitates comparative analyses within the large gene families of closely related species including the description of gene structure or expression profiles, as we demonstrated in our work. Obviously, different reference species must be used for the monocotyledonous plants that evolved the individual components of (not only) MSP signaling separately (Kaltenegger et al., 2018).
Homologs of ARR3, ARR6, and ARR16 are under evolutionary pressure against multiplication during Brassicaceae evolution
Gene or genome multiplication is an indispensable feature of plant evolution, and gene loss is a frequent fate of newly multiplicated genes (Lynch and Conery, 2000). More specifically, the majority of orthologous groups (~70%) in the common progenitor of recent Brassicaceae species Raphanus raphanistrum and B. rapa experienced losses after the WGT (Moghe et al., 2014). Interestingly, genes encoding individual MSP components (i.e. sensor HKs, HPts, and RRs) differ in the extent of gene loss and preservation during evolution. While in the case of HKs, gene loss is a dominant feature, response regulators, particularly RRAs are mostly preserved after WGDs (Kaltenegger et al., 2018).
In this context, we have rather surprisingly identified homologs of ARR3, ARR6, and ARR16 as singletons in both B. rapa and B. oleracea (Fig. 3), suggesting evolutionary pressure against the multiplication of those genes. The presence of two copies of the ARR3, ARR6, and ARR16 homologs in B. napus (a single copy in each subgenome) might be explained by the recency of the interploidization event. We confirmed the singleton status of ARR3, ARR6, and ARR16 orthologs also in other Brassicaceae species including diploid Camelina sativa and a single copy per subgenome in the allotetraploid Brassica juncea (Supplementary Figs S7, S8). The ability of the gene duplication to be retained seems to be associated with sequence and expression divergence, leading to functional diversification (Moghe et al., 2014). In our cytokinin and abiotic stress response assays, we did not observe any strong expression specificity of ARR3, ARR6, or ARR16 and their Brassica orthologs, potentially explaining the singleton status of those genes. In A. thaliana, some of the RRA genes were shown to play specific roles in controlling plant growth and development that cannot be explained solely by their functions as redundant cytokinin primary response genes and negative regulators of MSP signaling. To name a few, the ethylene-inducible ARR3 regulates RAM size (Zdarska et al., 2019) and is involved in the cytokinin-independent control over circadian rhythms (Salome et al., 2006). ARR6 mediates a negative interaction between abscisic acid and MSP signaling (Wang et al., 2011; Huang et al., 2017), plays a role in the CLE peptide-mediated inhibition of protoxylem formation (Kondo et al., 2011), and regulates pathogen immune response by controlling cell wall composition (Bacete et al., 2020). Finally, spatial-specific expression of ARR16 and ARR17 regulates the hydrotropic bending of the root (Chang et al., 2019), and controls stomata formation (Vaten et al., 2018) and leaf growth (Efroni et al., 2013). Thus, ARR3, ARR6, and ARR16 seem to mediate several key regulatory roles, which might be sensitive to gene dosage. To what extent the Brassica homologs of those genes play similar regulatory roles and whether this explains the observed negative selection, however, remains to be clarified.
Cytokinins contribute to abiotic stress-mediated induction of a subset of RRA genes
The A. thaliana RRA genes were originally described as cytokinin primary response genes, being rapidly (in the order of minutes) induced by exogenous cytokinin treatment (D’Agostino et al., 2000). Here, we categorized the RRA genes based on the kinetics of their cytokinin response into seven categories: (i) fast, (ii) fast-transient, (iii) down-regulated, (iv) later, (v) later transient, (vi) other, and (vii) no response. The corresponding transcriptional dynamics may reflect certain specificity within MSP signaling (Pekarova et al., 2016), with a possible impact on the downstream molecular network underlying the cytokinin cellular responses (Skalak et al., 2019). The proportion of individual RRA categories varied among tested species, with categories (iv) later, (v) later transient, (vi) other, and (vii) no response being specific for Brassica sp. However, a subset of RRA genes, including homologs of ARR5, ARR7, and ARR15 [all belonging to class (i) fast] exhibited comparable cytokinin responses in all the tested species. This observation, together with a high level of conservation of the DNA-binding GARP domain of RRBs and the cytokinin responsiveness of the TCSv2 reporter in B. rapa and B. napus, implies that RRA genes may share common features and functions within the Brassicaceae family. Interestingly, we observed that a subset of cytokinin-responsive RRA genes of the category (i) fast constitutes a core of the abiotic stress-responsive RRA genes. While homologs of ARR6, ARR7, and ARR15 were cold responsive, RRA genes similar to ARR7 and ARR15 (together with other RRA genes, particularly in B. napus) seem also to be involved in the response to salinity and high osmolarity in all the tested Brassica species, suggesting the existence of a common regulatory mechanism. This conclusion is also supported by the rapid induction of cytokinin reporter TCSv2 by all tested stress conditions. As the TCSv2 activation is solely RRB dependent, these data clearly support the involvement of MSP signaling in the abiotic stress response of Brassica crops. The TCS-based reporters were previously shown to reliably reflect the cytokinin signaling output in crops such as rice and tomato (Tao et al., 2017; Steiner et al., 2020). While the environmental conditions such as shade or osmotic stress significantly regulate TCS reporter activity in Arabidopsis (Novák et al., 2015; Rowe et al., 2016; Chang et al., 2019), no observation of stress-dependent regulation of the TCS system has been studied in crops so far. Thus, our results open up a new path facilitating further studies on the dynamics of signal transduction and stress adaptation in crops.
Our finding on the contribution of cytokinin signaling to the cold-mediated regulation of ARR7 and its Brassica homologs is in line with this hypothesis. (A)biotic stress has been shown to control endogenous hormone levels, including cytokinins, at the level of both biosynthesis and metabolism (Skalak et al., 2021, and references therein). This implies that stress-induced up-regulation of endogenous cytokinin levels might be a part of the cold (and probably other abiotic stress) response in Brassicaceae, thus further substantiating the proposed role of plant hormones as a regulatory interface between environmental conditions and intrinsic regulatory pathways controlling individual processes of plant growth and development (Ramireddy et al., 2014; Landrein et al., 2018; Cortleven et al., 2019; Skalak et al., 2021; Yamoune et al., 2021; Abualia et al., 2022; Waadt et al., 2022; Taleski et al., 2023).
Conclusions and future outlines
In summary, our work sheds light on the evolutionary relationships of MSP signaling within the Brassicaceae family. We provide a complete list of the RRA genes and their partial molecular characterization in the allotetraploid B. napus but also in its parental species, B. rapa and B. oleracea. That includes a novel classification reflecting the kinetics of their cytokinin-dependent transcriptional regulation. The conserved occurrence of ARR3, ARR6, and ARR16 and their orthologs as singletons in diploid members of the Brassicaceae family (A. thaliana, C. sativa, B. rapa, and B. oleracea) and a single copy per subgenome in allotetraploids B. napus and B. juncea implies the existence of gene-specific negative selection, possibly based on functional importance and preventing gene multiplication. Several of the RRA genes exhibited conserved expression patterns in response to cytokinin and abiotic stresses, implying the presence of common regulatory elements. Our data suggest that cold-mediated induction of RRA genes demands canonical cytokinin signaling in all tested Brassica species, thus emphasizing the importance of cytokinin-regulated MSP in abiotic stress responses. These findings contribute to a nuanced comprehension of the pivotal role of RRA genes in plant stress responses and open up novel avenues for further investigation to uncover the intricate mechanisms guiding plant growth and adaptation, with high potential for applied research. In this respect, the functional characterization of RRA genes, although challenging considering the redundancy previously observed in Arabidopsis (To et al., 2004), will be the next important goal in our efforts to elucidate their role in the abiotic crop response.
Supplementary data
The following supplementary data are available at JXB online.
Table S1. Gene and protein information on type-A response regulators from A. thaliana.
Table S2. Gene and protein information for Brassica RRA genes.
Table S3. List of primers used in the study.
Table S4. Relative expression of 1-week-old seedlings of A. thaliana, B. rapa, B. oleracea, and B. napus after cytokinin treatment.
Table S5. Arabidopsis-like cytokinin-responsive cis-elements identified in the promoter regions of A. thaliana and Brassica RRA genes.
Table S6. Relative expression of 1-week-old seedlings of A. thaliana, B. rapa, B. oleracea, and B. napus after exposure to abiotic stress treatment.
Table S7. Environmental stress-related cis-elements identified in the promoter regions of the type-A response regulators in A. thaliana, B. rapa, B. oleracea, and B. napus.
Table S8. Enrichment of stress-responsive transcription factors identified in the promoter regions of stress-responsive RRA genes in A. thaliana.
Table S9. Enrichment of stress-responsive transcription factors identified in the promoter regions of stress-responsive BrRRA, BoRRA, and BnRRA genes.
Fig. S1. Phylogenetic relationship of type-A response regulators in A. thaliana and B. rapa.
Fig. S2. Phylogenetic relationship of type-A response regulators in A. thaliana and B. oleracea.
Fig. S3. Phylogenetic relationship of type-A response regulators in A. thaliana and B. napus (A and C subgenome).
Fig. S4. The mean expression levels of B. rapa and B. oleracea RRA genes.
Fig. S5. The DNA-binding domains of type-B RRs are conserved in the Brassicaceae.
Fig. S6. Stress-responsive elements do not seem to control the expression of cold-responsive RRAs in Arabidopsis and Brassica sp.
Fig. S7. Phylogenetic relationship of RRAs in A. thaliana and Brassica juncea.
Fig. S8. Phylogenetic relationship of RRAs in A. thaliana and Camelina sativa.
Acknowledgements
We express our gratitude to the Crop Research Institute (Výzkumný ústav rostlinné výroby, v.v.i.) Genebank, Prague, Czech Republic, for providing the B. napus (Darmor) seeds. Additionally, we extend our acknowledgment to the core facility CELLIM, which is supported by MEYS CR (LM2023050 Czech-BioImaging), and the Plant Sciences Core Facility of CEITEC Masaryk University for their invaluable technical assistance.
Contributor Information
Katrina Leslie Nicolas Mala, CEITEC - Central European Institute of Technology, Masaryk University, Kamenice 5/A2, 625 00 Brno, Czech Republic; National Centre for Biomolecular Research, Faculty of Science, Masaryk University, Kamenice 5/A2, 625 00 Brno, Czech Republic.
Jan Skalak, CEITEC - Central European Institute of Technology, Masaryk University, Kamenice 5/A2, 625 00 Brno, Czech Republic; National Centre for Biomolecular Research, Faculty of Science, Masaryk University, Kamenice 5/A2, 625 00 Brno, Czech Republic.
Elena Zemlyanskaya, Institute of Cytology and Genetics, Siberian Branch, Russian Academy of Sciences, Novosibirsk, 630090, Russia; Faculty of Natural Sciences, Novosibirsk State University, Novosibirsk, 630090, Russia.
Vladislav Dolgikh, Institute of Cytology and Genetics, Siberian Branch, Russian Academy of Sciences, Novosibirsk, 630090, Russia; Faculty of Natural Sciences, Novosibirsk State University, Novosibirsk, 630090, Russia.
Veronika Jedlickova, CEITEC - Central European Institute of Technology, Masaryk University, Kamenice 5/A2, 625 00 Brno, Czech Republic.
Helene S Robert, CEITEC - Central European Institute of Technology, Masaryk University, Kamenice 5/A2, 625 00 Brno, Czech Republic.
Lenka Havlickova, Department of Biology, University of York, York, UK.
Klara Panzarova, PSI (Photon Systems Instruments), Ltd, Drásov, 66424 Drásov, Czech Republic.
Martin Trtilek, PSI (Photon Systems Instruments), Ltd, Drásov, 66424 Drásov, Czech Republic.
Ian Bancroft, Department of Biology, University of York, York, UK.
Jan Hejatko, CEITEC - Central European Institute of Technology, Masaryk University, Kamenice 5/A2, 625 00 Brno, Czech Republic; National Centre for Biomolecular Research, Faculty of Science, Masaryk University, Kamenice 5/A2, 625 00 Brno, Czech Republic.
Richard Napier, University of Warwick, UK.
Author contributions
JH: conceptualization and funding acquisition; JS and JH: coordination of the work; KLNM: performing all bioinformatic searches, ranging from BLAST to phylogenetic analysis, RT–qPCR, imaging of the transformed Brassica species with a cytokinin sensor, statistical analysis, and figure preparation, with assistance from JS; EZ and VD: promoter analysis, multiple sequence alignment of type-B RRs, and figure preparation; VJ and HSR: transformation with a cytokinin sensor and selection of the Brassica species; KP and MT: assistance with the stress response experiments; all authors wrote and revised the manuscript, and read and approved the final manuscript.
Conflict of interest
No conflict of interest declared.
Funding
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic, projects ‘TowArds Next GENeration Crops, TANGENC’ (CZ.02.01.01/00/22_008/0004581) and LUAUS24277. The work of VD was supported by the Russian State Budgetary Project (FWNR-2022-0006).
Data availability
All data supporting the findings of this study are available within the paper and its supplementary data published online.
References
- Abualia R, Otvos K, Novak O, Bouguyon E, Domanegg K, Krapp A, Nacry P, Gojon A, Lacombe B, Benkova E. 2022. Molecular framework integrating nitrate sensing in root and auxin-guided shoot adaptive responses. Proceedings of the National Academy of Sciences, USA 119, e2122460119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadi I, Novak O, Gelova Z, et al. 2020. Cell-surface receptors enable perception of extracellular cytokinins. Nature Communications 11, 4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura Y, Hagino T, Ohta Y, Aoki K, Yonekura-Sakakibara K, Deji A, Yamaya T, Sugiyama T, Sakakibara H. 2003. Molecular characterization of His–Asp phosphorelay signaling factors in maize leaves: implications of the signal divergence by cytokinin-inducible response regulators in the cytosol and the nuclei. Plant Molecular Biology 52, 331–341. [DOI] [PubMed] [Google Scholar]
- Bacete L, Melida H, Lopez G, Dabos P, Tremousaygue D, Denance N, Miedes E, Bulone V, Goffner D, Molina A. 2020. Arabidopsis Response Regulator 6 (ARR6) modulates plant cell-wall composition and disease resistance. Molecular Plant-Microbe Interactions 33, 767–780. [DOI] [PubMed] [Google Scholar]
- Bartlett A, O’Malley RC, Huang SSC, Galli M, Nery JR, Gallavotti A, Ecker JR. 2017. Mapping genome-wide transcription-factor binding sites using DAP-seq. Nature Protocols 12, 1659–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar A, Paul LK, Sharma E, Jha S, Jain M, Khurana JP. 2021. OsRR6, a type-A response regulator in rice, mediates cytokinin, light and stress responses when over-expressed in Arabidopsis. Plant Physiology and Biochemistry 161, 98–112. [DOI] [PubMed] [Google Scholar]
- Buechel S, Leibfried A, To JP, Zhao Z, Andersen SU, Kieber JJ, Lohmann JU. 2010. Role of A-type ARABIDOPSIS RESPONSE REGULATORS in meristem maintenance and regeneration. European Journal of Cell Biology 89, 279–284. [DOI] [PubMed] [Google Scholar]
- Chang J, Li X, Fu W, et al. 2019. Asymmetric distribution of cytokinins determines root hydrotropism in Arabidopsis thaliana. Cell Research 29, 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao JT, Kong YZ, Wang Q, Sun YH, Gong DP, Lv J, Liu GS. 2015. MapGene2Chrom, a tool to draw gene physical map based on Perl and SVG languages. Yi Chuan 37, 91–97. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. 2020. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Molecular Plant 13, 1194–1202. [DOI] [PubMed] [Google Scholar]
- Chen H, Wang T, He X, Cai X, Lin R, Liang J, Wu J, King G, Wang X. 2021. BRAD V3.0: an upgraded Brassicaceae database. Nucleic Acids Research 50, D1432–D1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Wu J, Fang L, Wang X. 2012. Syntenic gene analysis between Brassica rapa and other Brassicaceae species. Frontiers in Plant Science 3, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Wu J, Wang X. 2014. Genome triplication drove the diversification of Brassica plants. Horticulture Research 1, 14024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortleven A, Leuendorf JE, Frank M, Pezzetta D, Bolt S, Schmulling T. 2019. Cytokinin action in response to abiotic and biotic stresses in plants. Plant, Cell & Environment 42, 998–1018. [DOI] [PubMed] [Google Scholar]
- Cunningham F, Allen JE, Allen J, et al. 2021. Ensembl 2022. Nucleic Acids Research 50, D988–D995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino IB, Deruere J, Kieber JJ. 2000. Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiology 124, 1706–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoon MJL, Imoto S, Nolan J, Miyano S. 2004. Open source clustering software. Bioinformatics 20, 1453–1454. [DOI] [PubMed] [Google Scholar]
- Duvaud S, Gabella C, Lisacek F, Stockinger H, Ioannidis V, Durinx C. 2021. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Research 49, W216–W227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efroni I, Han SK, Kim HJ, Wu MF, Steiner E, Birnbaum KD, Hong JC, Eshed Y, Wagner D. 2013. Regulation of leaf maturation by chromatin-mediated modulation of cytokinin responses. Developmental Cell 24, 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission. 2019. Oilseeds and protein crops production. https://agridata.ec.europa.eu/extensions/DataPortal/oilseeds-protein-crops.html [Google Scholar]
- Guénin S, Mauriat M, Pelloux J, Van Wuytswinkel O, Bellini C, Gutierrez L. 2009. Normalization of qRT-PCR data: the necessity of adopting a systematic, experimental conditions-specific, validation of references. Journal of Experimental Botany 60, 487–493. [DOI] [PubMed] [Google Scholar]
- Havlickova L, He Z, Wang L, Langer S, Harper AL, Kaur H, Broadley MR, Gegas V, Bancroft I. 2018. Validation of an updated associative transcriptomics platform for the polyploid crop species Brassica napus by dissection of the genetic architecture of erucic acid and tocopherol isoform variation in seeds. The Plant Journal 93, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. 2010. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Molecular Cell 38, 576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejatko J, Ryu H, Kim GT, et al. 2009. The histidine kinases CYTOKININ-INDEPENDENT1 and ARABIDOPSIS HISTIDINE KINASE2 and 3 regulate vascular tissue development in Arabidopsis shoots. The Plant Cell 21, 2008–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. 2000. pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Molecular Biology 42, 819–832. [DOI] [PubMed] [Google Scholar]
- Hendriks KP, Kiefer C, Al-Shehbaz IA, et al. 2023. Global Brassicaceae phylogeny based on filtering of 1,000-gene dataset. Current Biology 33, 4052–4068. [DOI] [PubMed] [Google Scholar]
- Heyl A, Brault M, Frugier F, Kuderova A, Lindner AC, Motyka V, Rashotte AM, Schwartzenberg KV, Vankova R, Schaller GE. 2013. Nomenclature for members of the two-component signaling pathway of plants. Plant Physiology 161, 1063–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda K, Imamura A, Katoh E, Hatta T, Tachiki M, Yamada H, Mizuno T, Yamazaki T. 2002. Molecular structure of the GARP family of plant Myb-related DNA binding motifs of the Arabidopsis response regulators. The Plant Cell 14, 2015–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe KL, Achuthan P, Allen J, et al. 2021. Ensembl 2021. Nucleic Acids Research 49, D884–D891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. 2015. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31, 1296–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhang X, Gong Z, Yang S, Shi Y. 2017. ABI4 represses the expression of type-A ARRs to inhibit seed germination in Arabidopsis. The Plant Journal 89, 354–365. [DOI] [PubMed] [Google Scholar]
- Hwang I, Chen HC, Sheen J. 2002. Two-component signal transduction pathways in Arabidopsis. Plant Physiology 129, 500–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sheen J. 2001. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413, 383–389. [DOI] [PubMed] [Google Scholar]
- Imamura A, Hanaki N, Umeda H, Nakamura A, Suzuki T, Ueguchi C, Mizuno T. 1998. Response regulators implicated in His-to-Asp phosphotransfer signaling in Arabidopsis. Proceedings of the National Academy of Sciences, USA 95, 2691–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura A, Kiba T, Tajima Y, Yamashino T, Mizuno T. 2003. In vivo and in vitro characterization of the ARR11 response regulator implicated in the His-to-Asp phosphorelay signal transduction in Arabidopsis thaliana. Plant and Cell Physiology 44, 122–131. [DOI] [PubMed] [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T. 2001. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409, 1060–1063. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kurata N. 2006. Identification and characterization of cytokinin-signalling gene families in rice. Gene 382, 57–65. [DOI] [PubMed] [Google Scholar]
- Jain M, Tyagi AK, Khurana JP. 2006. Molecular characterization and differential expression of cytokinin-responsive type-A response regulators in rice (Oryza sativa). BMC Plant Biology 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlickova V, Macova K, Stefkova M, Butula J, Stavenikova J, Sedlacek M, Robert HS. 2022. Hairy root transformation system as a tool for CRISPR/Cas9-directed genome editing in oilseed rape (Brassica napus). Frontiers in Plant Science 13, 919290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J, Kim NY, Kim S, et al. 2010. A subset of cytokinin two-component signaling system plays a role in cold temperature stress response in Arabidopsis. Journal of Biological Chemistry 285, 23371–23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JJ, Li N, Chen WJ, Wang Y, Rong H, Xie T, Wang YP. 2022. Genome-wide analysis of the type-B authentic response regulator gene family in Brassica napus. Genes 13, 1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenegger E, Leng S, Heyl A. 2018. The effects of repeated whole genome duplication events on the evolution of cytokinin signaling pathway. BMC Evolutionary Biology 18, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang NY, Cho C, Kim NY, Kim J. 2012. Cytokinin receptor-dependent and receptor-independent pathways in the dehydration response of Arabidopsis thaliana. Journal of Plant Physiology 169, 1382–1391. [DOI] [PubMed] [Google Scholar]
- Karan R, Singla-Pareek SL, Pareek A. 2009. Histidine kinase and response regulator genes as they relate to salinity tolerance in rice. Functional & Integrative Genomics 9, 411–417. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Schaller GE. 2018. Cytokinin signaling in plant development. Development 145, dev149344. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Hirakawa Y, Kieber JJ, Fukuda H. 2011. CLE peptides can negatively regulate protoxylem vessel formation via cytokinin signaling. Plant and Cell Physiology 52, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubiasova K, Montesinos JC, Samajova O, et al. 2020. Cytokinin fluoroprobe reveals multiple sites of cytokinin perception at plasma membrane and endoplasmic reticulum. Nature Communications 11, 4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderova A, Gallova L, Kuricova K, Nejedla E, Curdova A, Micenkova L, Plihal O, Smajs D, Spichal L, Hejatko J. 2015. Identification of AHK2- and AHK3-like cytokinin receptors in Brassica napus reveals two subfamilies of AHK2 orthologues. Journal of Experimental Botany 66, 339–353. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sharma P, Thomas L, Agnihotri A, Banga S. 2009. Canola cultivation in India: scenario and future strategy. In: 16th Australian research assembly on Brassicas. Ballarat, Victoria.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann P, Mayer BE, Hamacher K. 2020. Substitution matrix based color schemes for sequence alignment visualization. BMC Bioinformatics 21, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrein B, Formosa-Jordan P, Malivert A, Schuster C, Melnyk CW, Yang W, Turnbull C, Meyerowitz EM, Locke JCW, Jonsson H. 2018. Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proceedings of the National Academy of Sciences, USA 115, 1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Kim S, Ha YM, Kim J. 2008. Phosphorylation of Arabidopsis response regulator 7 (ARR7) at the putative phospho-accepting site is required for ARR7 to act as a negative regulator of cytokinin signaling. Planta 227, 577–587. [DOI] [PubMed] [Google Scholar]
- Leibfried A, To JP, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU. 2005. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438, 1172–1175. [DOI] [PubMed] [Google Scholar]
- Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. 2002. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research 30, 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZN, Zhang M, Kong LJ, Lv YX, Zou MH, Lu G, Cao JS, Yu XL. 2014. Genome-wide identification, phylogeny, duplication, and expression analyses of two-component system genes in Chinese cabbage (Brassica rapa ssp pekinensis). DNA Research 21, 379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrmann J, Sweere U, Zabaleta E, Baurle I, Keitel C, Kozma-Bognar L, Brennicke A, Schafer E, Kudla J, Harter K. 2001. The response regulator ARR2: a pollen-specific transcription factor involved in the expression of nuclear genes for components of mitochondrial Complex I in Arabidopsis. Molecular Genetics and Genomics 265, 2–13. [DOI] [PubMed] [Google Scholar]
- Lynch M, Conery JS. 2000. The evolutionary fate and consequences of duplicate genes. Science 290, 1151–1155. [DOI] [PubMed] [Google Scholar]
- Lysak MA, Koch MA, Pecinka A, Schubert I. 2005. Chromosome triplication found across the tribe Brassiceae. Genome Research 15, 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira FA-O, Pearce MA-O, Tivey ARN, Basutkar P, Lee J, Edbali O, Madhusoodanan N, Kolesnikov A, Lopez RA-O. 2022. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Research 50, W276–W279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Mathews DE, Argyros DA, Maxwell BB, Kieber JJ, Alonso JM, Ecker JR, Schaller GE. 2005. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. The Plant Cell 17, 3007–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida K, Yoshida T, Sakurai T, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS. 2010. Genome-wide analysis of two-component systems and prediction of stress-responsive two-component system members in soybean. DNA Research 17, 303–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghe GD, Hufnagel DE, Tang H, Xiao Y, Dworkin I, Town CD, Conner JK, Shiu SH. 2014. Consequences of whole-genome triplication as revealed by comparative genomic analyses of the wild radish Raphanus raphanistrum and three other Brassicaceae species. The Plant Cell 26, 1925–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga T. 1929. lnterspecific hybridization in Brassica. The cytology of Fl hybrids of B. napella and various other species with 10 chromosomes. Cytologia 1, 16–27. [Google Scholar]
- Muller B, Sheen J. 2007. Advances in cytokinin signaling. Science 318, 68–69. [DOI] [PubMed] [Google Scholar]
- Muller B, Sheen J. 2008. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453, 1094–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaharu U, Nagaharu N. 1935. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Journal of Japanese Botany 7, 389–452. [Google Scholar]
- Nikolov LA, Shushkov P, Nevado B, Gan X, Al-Shehbaz IA, Filatov D, Bailey CD, Tsiantis M. 2019. Resolving the backbone of the Brassicaceae phylogeny for investigating trait diversity. New Phytologist 222, 1638–1651. [DOI] [PubMed] [Google Scholar]
- Novák J, Černý M, Pavlů J, Zemánková J, Skalák J, Plačková L, Brzobohatý B. 2015. Roles of proteome dynamics and cytokinin signaling in root to hypocotyl ratio changes induced by shading roots of Arabidopsis seedlings. Plant and Cell Physiology 56, 1006–1018. [DOI] [PubMed] [Google Scholar]
- Okonechnikov K, Golosova O, Fursov M, UGENE Team. 2012. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 28, 1166–1167. [DOI] [PubMed] [Google Scholar]
- Pareek A, Singh A, Kumar M, Kushwaha HR, Lynn AM, Singla-Pareek SL. 2006. Whole-genome analysis of Oryza sativa reveals similar architecture of two-component signaling machinery with Arabidopsis. Plant Physiology 142, 380–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarova B, Szmitkowska A, Dopitova R, Degtjarik O, Zidek L, Hejatko J. 2016. Structural aspects of multistep phosphorelay-mediated signaling in plants. Molecular Plant 9, 71–85. [DOI] [PubMed] [Google Scholar]
- Pernisova M, Klima P, Horak J, et al. 2009. Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proceedings of the National Academy of Sciences, USA 106, 3609–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. 2004. Quantification strategies in real-time PCR. In: Bustin SA, ed. A-Z of quantitative PCR. La Jolla, CA: International University Line, 87–112. [Google Scholar]
- Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramireddy E, Chang L, Schmulling T. 2014. Cytokinin as a mediator for regulating root system architecture in response to environmental cues. Plant Signaling & Behavior 9, e27771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore SS, Babu S, Shekhawat K, Singh VK, Upadhyay PK, Singh RK, Raj R, Singh H, Zaki FM. 2022. Oilseed Brassica species diversification and crop geometry influence the productivity, economics, and environmental footprints under semi-arid regions. Sustainability 14, 2230. [Google Scholar]
- Rowe JH, Topping JF, Liu J, Lindsey K. 2016. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytologist 211, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. 1987. The Neighbor–Joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Sakai H, Aoyama T, Oka A. 2000. Arabidopsis ARR1 and ARR2 response regulators operate as transcriptional activators. The Plant Journal 24, 703–711. [DOI] [PubMed] [Google Scholar]
- Saldanha AJ. 2004. Java Treeview—extensible visualization of microarray data. Bioinformatics 20, 3246–3248. [DOI] [PubMed] [Google Scholar]
- Salome PA, To JP, Kieber JJ, McClung CR. 2006. Arabidopsis response regulators ARR3 and ARR4 play cytokinin-independent roles in the control of circadian period. The Plant Cell 18, 55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller GE, Kieber JJ, Shiu SH. 2008. Two-component signaling elements and histidyl-aspartyl phosphorelays. Arabidopsis Book 6, e0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharan A, Soni P, Nongpiur RC, Singla-Pareek SL, Pareek A. 2017. Mapping the ‘Two-component system’ network in rice. Scientific Reports 7, 9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Tian S, Hou L, Huang X, Zhang X, Guo H, Yang S. 2012. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-A ARR genes in Arabidopsis. The Plant Cell 24, 2578–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalak J, Nicolas KL, Vankova R, Hejatko J. 2021. Signal integration in plant abiotic stress responses via multistep phosphorelay signaling. Frontiers in Plant Science 12, 644823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalak J, Vercruyssen L, Claeys H, et al. 2019. Multifaceted activity of cytokinin in leaf development shapes its size and structure in Arabidopsis. The Plant Journal 97, 805–824. [DOI] [PubMed] [Google Scholar]
- Spichal L, Werner T, Popa I, Riefler M, Schmulling T, Strnad M. 2009. The purine derivative PI-55 blocks cytokinin action via receptor inhibition. The FEBS Journal 276, 244–253. [DOI] [PubMed] [Google Scholar]
- Steiner E, Israeli A, Gupta R, et al. 2020. Characterization of the cytokinin sensor TCSv2 in arabidopsis and tomato. Plant Methods 16, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Lv L, Zhao J, et al. 2022. Genome-wide identification and expression analysis of the TaRRA gene family in wheat (Triticum aestivum L.). Frontiers in Plant Science 13, 1006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleski M, Chapman K, Novak O, Schmulling T, Frank M, Djordjevic MA. 2023. CEP peptide and cytokinin pathways converge on CEPD glutaredoxins to inhibit root growth. Nature Communications 14, 1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Kiba T, Sakakibara H, Ueguchi C, Mizuno T, Sugiyama T. 1998. Expression of Arabidopsis response regulator homologs is induced by cytokinins and nitrate. FEBS Letters 429, 259–262. [DOI] [PubMed] [Google Scholar]
- Tao J, Sun H, Gu P, Liang Z, Chen X, Lou J, Xu G, Zhang Y. 2017. A sensitive synthetic reporter for visualizing cytokinin signaling output in rice. Plant Methods 13, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To JP, Haberer G, Ferreira FJ, Deruere J, Mason MG, Schaller GE, Alonso JM, Ecker JR, Kieber JJ. 2004. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. The Plant Cell 16, 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touzet H, Varré J-S. 2007. Efficient and accurate P-value computation for Position Weight Matrices. Algorithms for Molecular Biology 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town CD, Cheung F, Maiti R, et al. 2006. Comparative genomics of Brassica oleracea and Arabidopsis thaliana reveal gene loss, fragmentation, and dispersal after polyploidy. The Plant Cell 18, 1348–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran LS, Urao T, Qin F, Maruyama K, Kakimoto T, Shinozaki K, Yamaguchi-Shinozaki K. 2007. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proceedings of the National Academy of Sciences, USA 104, 20623–20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Weir NR, Hill K, Zhang W, Kim HJ, Shiu SH, Schaller GE, Kieber JJ. 2012. Characterization of genes involved in cytokinin signaling and metabolism from rice. Plant Physiology 158, 1666–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T, Yakubov B, Yamaguchi-Shinozaki K, Shinozaki K. 1998. Stress-responsive expression of genes for two-component response regulator-like proteins in Arabidopsis thaliana. FEBS Letters 427, 175–178. [DOI] [PubMed] [Google Scholar]
- Vaten A, Soyars CL, Tarr PT, Nimchuk ZL, Bergmann DC. 2018. Modulation of asymmetric division diversity through cytokinin and SPEECHLESS regulatory interactions in the arabidopsis stomatal lineage. Developmental Cell 47, 53–66.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Seller CA, Hsu PK, Takahashi Y, Munemasa S, Schroeder JI. 2022. Plant hormone regulation of abiotic stress responses. Nature Reviews. Molecular Cell Biology 23, 680–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Lin TC, Kieber J, Tsai YC. 2019. Response regulator 9 and 10 negatively regulate salinity tolerance in rice. Plant and Cell Physiology 60, 2549–2563. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li L, Ye T, Zhao S, Liu Z, Feng YQ, Wu Y. 2011. Cytokinin antagonizes ABA suppression to seed germination of Arabidopsis by downregulating ABI5 expression. The Plant Journal 68, 249–261. [DOI] [PubMed] [Google Scholar]
- Werner T, Schmulling T. 2009. Cytokinin action in plant development. Current Opinion in Plant Biology 12, 527–538. [DOI] [PubMed] [Google Scholar]
- Xie M, Chen H, Huang L, O’Neil RC, Shokhirev MN, Ecker JR. 2018. A B-ARR-mediated cytokinin transcriptional network directs hormone cross-regulation and shoot development. Nature Communications 9, 1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamoune A, Cuyacot AR, Zdarska M, Hejatko J. 2021. Hormonal orchestration of root apical meristem formation and maintenance in Arabidopsis. Journal of Experimental Botany 72, 6768–6788. [DOI] [PubMed] [Google Scholar]
- Yang TJ, Kim JS, Kwon SJ, et al. 2006. Sequence-level analysis of the diploidization process in the triplicated FLOWERING LOCUS C region of Brassica rapa. The Plant Cell 18, 1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates AA-O, Allen J, Amode RM, et al. 2022. Ensembl Genomes 2022: an expanding genome resource for non-vertebrates. Nucleic Acids Research 50, D996–D1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdarska M, Cuyacot AR, Tarr PT, Yamoune A, Szmitkowska A, Hrdinova V, Gelova Z, Meyerowitz EM, Hejatko J. 2019. ETR1 integrates response to ethylene and cytokinins into a single multistep phosphorelay pathway to control root growth. Molecular Plant 12, 1338–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Liu T, Li X, Duan M, Wang J, Qiu Y, Wang H, Song J, Shen D. 2016. Interspecific hybridization, polyploidization and backcross of Brassica oleracea var. alboglabra with B. rapa var. purpurea morphologically recapitulate the evolution of Brassica vegetables. Scientific Reports 6, 18618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, Lohmann JU. 2010. Hormonal control of the shoot stem-cell niche. Nature 465, 1089–1092. [DOI] [PubMed] [Google Scholar]
- Zubo YO, Blakley IC, Yamburenko MV, et al. 2017. Cytokinin induces genome-wide binding of the type-B response regulator ARR10 to regulate growth and development in Arabidopsis. Proceedings of the National Academy of Sciences, USA 114, E5995–E6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerkandl E, Pauling L. 1965. Evolutionary divergence and convergence in proteins. In: Bryson V, Vogel HJ, eds. Evolving genes and proteins. Academic Press, 97–166. [Google Scholar]
- Zurcher E, Muller B. 2016. Cytokinin synthesis, signaling, and function—advances and new insights. International Review of Cell and Molecular Biology 324, 1–38. [DOI] [PubMed] [Google Scholar]
- Zurcher E, Tavor-Deslex D, Lituiev D, Enkerli K, Tarr PT, Muller B. 2013. A robust and sensitive synthetic sensor to monitor the transcriptional output of the cytokinin signaling network in planta. Plant Physiology 161, 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its supplementary data published online.