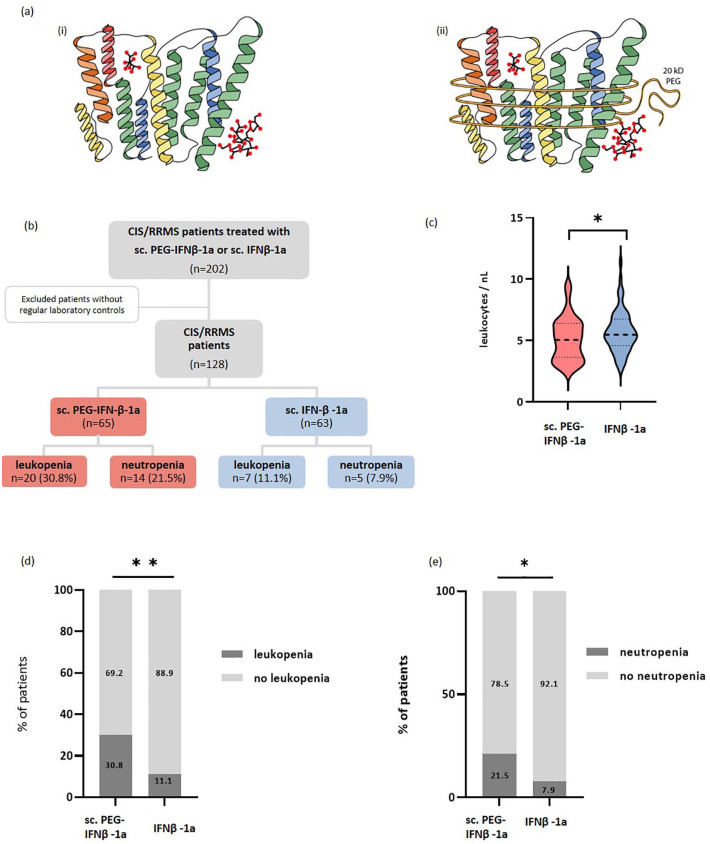
Figure 1.
Biochemical structure and study design. (a) The biochemical structure of (i) Rebif® and (ii) Plegridy®; sc. IFN-β-1a has the same amino acid sequence as human IFN-β and is glycosylated. The difference between the two formulations results from the addition of a non-toxic polymer to the α-amino group of the N-terminus of IFN-β-1, the so-called methoxy-PEG-O2-methylpropionaldehyde. (b) Study design: Out of a total of 128 patients with CIS or RRMS, 65 were treated with sc. PEG-IFN-β-1a and 63 were treated with sc. IFN-β-1a. (c) The absolute leukocyte count was significantly lower under treatment with sc. PEG-IFN-β-1a (red) compared to sc. IFN-β-1a (blue) (p = 0.046). The incidence of (d) leukopenia (p = 0.006) and (e) neutropenia (p = 0.03) was significantly higher in patients treated with sc. PEG-IFN-β-1a compared to patients treated with sc. IFN-β-1a.
*p < 0.05.**p < 0.01.
CIS, clinically isolated syndrome; IFN-β, interferon-beta; RRMS, relapsing-remitting multiple sclerosis.