Figure 7.
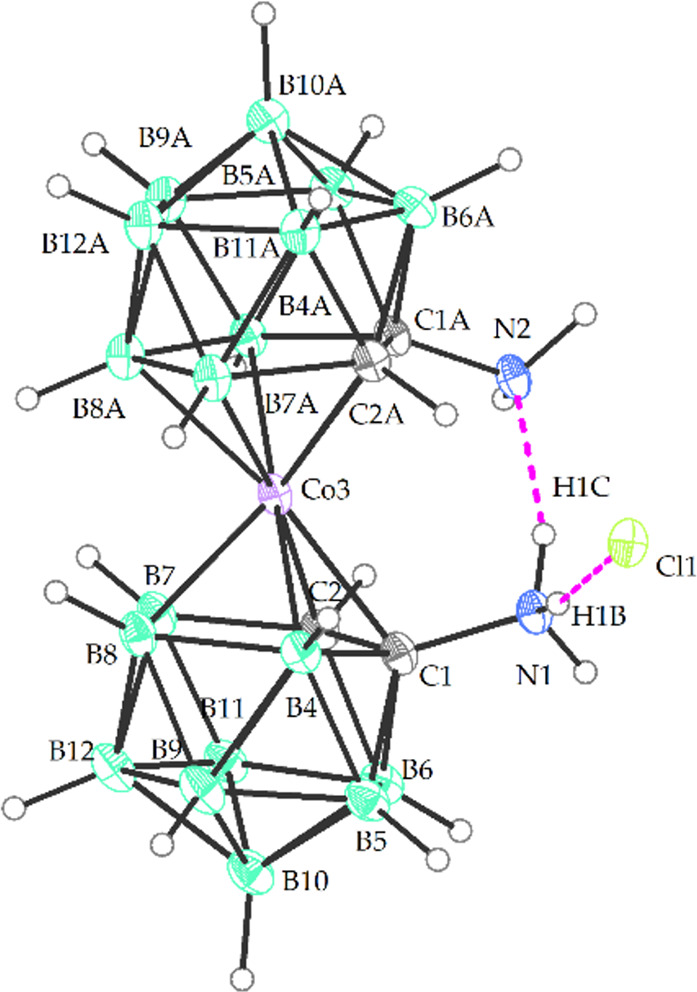
Crystal structure of the protonated form of the diamine Me4N5.HCl (ORTEP view, 30%). The solvating acetone molecule and the Me4N+ cation have been omitted for clarity. Selected interatomic distances [Å] and angles [°]: C1 N1 1.466(4), C1A N2 1.442(4), C1 C2 1.621(4), C1A C2A 1.639(4), C1 B4 1.698(5), C1A B4A 1.703(5), C2 B7 1.716(5), C2A C7A 1.714(5), C1 B5 1.696(5),1.711(5), C2 Co3 2.064(3), C1A Co3 2.103(3), Co3 B4 2.102(4), Co3 B7A 2.073(4), Co3 B7 2.093(4), Co3 B8 2.119(4), Co3 B8A 2.108(4), C2 C1 N1 119.1(3), C2A C1A N2 117.6(3), N1 C1 B4 122.0(3), N2 C1A B4A 124.8(3), N1 C1 B6 111.1(3), N2 C1A B6A 111.4(3), N1 C1 B5 115.1(3), N2 C1A B5A 118.4(3), C1 Co3 C1A 104.46(13), C1 Co3 C2A 104.28(13), C2 Co3 C2A 137.17(13), C1 C2 B7 112.1(3), C1A C2A B7A 112.8(2).
