Abstract
Background
Multiple models intravoxel incoherent motion (IVIM) based 18F-fluorodeoxyglucose positron emission tomography-magnetic resonance(18F-FDG PET/MR) could reflect the microscopic information of the tumor from multiple perspectives. However, its value in the prognostic assessment of non-small cell lung cancer (NSCLC) still needs to be further explored.
Objective
To compare the value of 18F-FDG PET/MR metabolic parameters and diffusion parameters in the prognostic assessment of patients with NSCLC.
Meterial and methods
Chest PET and IVIM scans were performed on 61 NSCLC patients using PET/MR. The maximum standard uptake value (SUVmax), metabolic tumor volume (MTV), total lesion glycolysis (TLG), diffusion coefficient (D), perfusion fraction (f), pseudo diffusion coefficient (D*) and apparent diffusion coefficient (ADC) were calculated. The impact of SUVmax, MTV, TLG, D, f, D*and ADC on survival was measured in terms of the hazard ratio (HR) effect size. Overall survival time (OS) and progression-free survival time (PFS) were evaluated with the Kaplan–Meier and Cox proportional hazard models. Log-rank test was used to analyze the differences in parameters between groups.
Results
61 NSCLC patients had an overall median OS of 18 months (14.75, 22.85) and a median PFS of 17 months (12.00, 21.75). Univariate analysis showed that pathological subtype, TNM stage, surgery, SUVmax, MTV, TLG, D, D* and ADC were both influential factors for OS and PFS in NSCLC patients. Multifactorial analysis showed that MTV, D* and ADC were independent predicting factors for OS and PFS in NSCLC patients.
Conclusion
MTV, D* and ADC are independent predicting factors affecting OS and PFS in NSCLC patients. 18F-FDG PET/MR-derived metabolic parameters and diffusion parameters have clinical value for prognostic assessment of NSCLC patients.
Keywords: Non-small cell lung cancer, Metabolic parameters, Diffusion parameters, Positron emission computed tomography, Magnetic resonance imaging
Introduction
Lung cancer is one of the most common cancers worldwide and the leading cause of cancer deaths in both developed and developing countries [1]. About 80% of the lung cancer patients are diagnosed with non-small cell lung cancer (NSCLC), and their 5-year survival rate is only 18% [1, 2]. Recent medical advances have stimulated a huge increase in overall cancer survival, but this improvement has not been ideal for lung cancer because most NSCLC has a relatively poor prognosis. Studies have shown that early assessment of prognostic factors in NSCLC patients is of great significance in determining and adjusting treatment plans, improving patient outcomes and enhancing patient quality of life [3].
Positron emission tomography-computed tomography(PET/CT) was often used to evaluate lung cancer because it was noninvasive and could provide both metabolic and morphological information about the lesion at the same time [4]. Maximum standard uptake value(SUVmax) represents the highest metabolic value in the lesions, and some studies found that SUVmax can predict the prognosis of NSCLC [5, 6]. This maybe because tumors with higher pathological grade and higher stage are more heterogeneity, and cell multiplication is usually more rapid and therefore their glucose uptake is higher. Metabolic tumor volume(MTV) and total lesion glycolysis(TLG) can integrate the volume of the lesion with the metabolism, which could reflect the overall metabolism of the tumor more sufficient.
With the introduction of the concept of molecular imaging, molecular imaging techniques represented by PET magnetic resonance (PET/MR) have shown their unique advantages in displaying characeristic lesions at the tissue, reflecting changes at the molecular level in the living organism in cellular and subcellular levels, and evaluating the features of the target area qualitatively and quantitatively. In contrast to CT, PET/MR has no radiation and it can provide multiple functional parameters along with morphological and metabolic information about the focus. Meanwhile application of quantitative and semi-quantitative indicators to predict early tumor response to therapy has received most attention [7–9]. Diffusion-weighted imaging (DWI) can reflect the microstructure of tissues by obtaining motion information of water molecules. The apparent diffusion coefficient (ADC) is a quantitative indicator of the degree of diffusion limitation in DWI response. ADC values inversely correlate with cellularity, it can reflect the diffusion of water molecules in biological tissues and is usually obvious lower in malignant tumors than normal tissues and benign lesions. Its value in distinguishing benign and malignant lesions in the lung and mediastinum is well documented, however, few articles have used it to evaluate the prognosis of lung cancer [10, 11].
The MRI technique known as Intravoxel incoherent motion (IVIM) was initially introduced by Le Bihan et al. [12]. They realized the separation of tissue water molecule diffusion from blood vessel water molecule diffusion, making up for the shortage of DWI. The most important advantage of IVIM is that, as a non-contrast perfusion imaging method, it can be used in some patients with contrast contraindications. The measurement parameters are as follows: (1) the diffusion coefficient (D), which reflects the diffusion of water molecules in interstitial tissues; (2) the perfusion fraction (f), which reflects the proportion of blood perfusion to total diffusion; and (3) the pseudo diffusion coefficient (D*), is a false diffusion caused by blood microcirculation and belongs to rapid diffusion [13]. IVIM has already been applied to study a number of disease, including the breast cancer [14], cervical cancer [15], and prostatic cancer [16]. In this study, 18F-fluorodeoxyglucose (18F-FDG) PET/MR was performed to assess the prognostic value of SUVmax, MTV, TLG and diffusion parameter D, f, D* and ADC in patients with NSCLC. As far as we know, these parameters are rarely used in the study of lung cancer prognosis, so our study is of great significance.
Patients and methods
Case selection and general information
This study was approved by the Ethics Committee of Henan Provincial People's hospital (2021 Lungren Trial No. 148) and each patient's written informed consent was obtained. From July 2020 to October 2021, a total of 135 patients with pulmonary lumps or nodules diagnosed by chest CT underwent chest PET/MRI. Two radiologists (N M and FF F with 10 and 15 years of experience in imaging diagnosis, respectively), blined to the clinical and pathological information of patients, interpreted MR and PET images. The inclusion criteria were as follows: 1. Diagnosis of NSCLC confirmed by cytology or histopathology; 2. The lesions were not treated with invasive treatment, radiotherapy or chemotherapy before PET/MRI examination; 3. Clinical data of patients were complete and follow-up information could be provided. The following patients were excluded: 1. Combination of severe heart, liver, kidney and hematopoietic diseases, severe lung dysfunction and other malignancies (n = 18); 2. Solid lesion component cross-sectional diameter < 10 mm (n = 14); 3. No histopathological results (n = 22); 4. Poor image quality or incomplete image sequences (n = 20). Finally, a total of 61 patients (29 males and 32 females, age range 40–81 years) were included in this study. Analyses covered age, sex, smoking status, subtype, stage, lesion diameter, and related parameters.
Imaging procotal
All patients underwent chest scanning with an integrated 3.0 T PET/MR (uPMR790, United Imaging Medical, Shanghai, China) and a 12-channel body coil. Fasting blood glucose level were < 8.0 mmol/L after at least 6 h of fasting before the examination (diabetic patients should take oral hypoglycemic drugs as prescribed to ensure fasting blood glucose < 8.0 mmol/L). 18F-FDG is produced by FracerLab FX-FDG (GE Minitrac) with a purity of over 95% and a pH value between 4.5 and 8.5. After intravenous injection of the tracer with a dose of 0.11 mCi/kg and resting for 1 h, the patient underwent a PET scan in a supine position, connected to a breathing belt to monitor breathing, with a scanning range from the apex of the lung to the angle of the diaphragm. The Dixon MRI sequence was used in PET scanning to attenuate gamma rays, and the ordered subset maximum expected (OSME) iterative method was used for image reconstruction. Upload the data to the workstation for post-processing. Simultaneously with PET scan (27 min), axial MR based magnetic resonance attenuation correction (MRAC), T2 weighted imaging (T2WI), T1 weighted imaging (T1WI), IVIM, and DWI were performed sequentially [17]. The summary of MRI acquisition parameters is shown in Table 1.
Table 1.
Imaging protocol parameters
| Parameters | MRAC | T2WI | T1WI | DWI | IVIM |
|---|---|---|---|---|---|
| TR (msec) | 4.92 | 3315 | 4.24 | 1620 | 1620 |
| TE (msec) | 2.24 | 87.8 | 1.13 | 69.8 | 69.6 |
| FOV (mm2) | 500 × 350 | 500 × 350 | 500 × 350 | 500 × 350 | 500 × 350 |
| Matrix | 192 × 192 | 320 × 70 | 320 × 70 | 128 × 100 | 128 × 100 |
| Slice thickness (mm) | 2 | 5 | 6 | 5 | 5 |
| Scanning time | 2min4s | 2min26s | 14 s | 40 s | 3min38s |
| b-values (s/mm2) | / | / | / | 0, 1000 | 0, 25, 50, 100, 150, 200, 400, 600, 800 and 1000 |
| Breath control | Free | Navigation | Holding | Free | Free |
| Orientation | Axial | Axial | Axial | Axial | Axial |
MRAC MR-base attenuation correction, T2WI T2-weighted imaging, T1WI T1-weighted imaging, DWI Diffusion weighted imaging, IVIM Intravoxel incoherent motion, TR/TE Repetition time/echo time, FOV Field of view
Imaging processing
All PET/MR images were imported into a United Imaging Healthcare (UIH) workstation (uWS-MR: R005) for aftertreatment and measurement. Two radiologists (H J and ZQ L with more than 5 years of experience in imaging diagnosis),, blined to the clinical and pathological information of patients, manually delineated the regions of interest (ROIs) to allow measurement of DWI and IVIM parameters.When the patient had multiple lesions, we selected the primary lesion as the target lesion and measured its related parameters. When delineating the ROIs, the observers were required to avoid blood vessels, trachea, necrosis and bleeding to ensure selection of the solid region with uniform component [17]. The average value measured by two observers was used for statistical analysis. If necessary, solve discrepancies with the help of a third radiologist. Lesions were found on the IVIM pseudo-color map, and ROI was mapped to measure D, f, and D* values. SUVmax and MTV were automatically calculated by the software, which automatically covers the whole lesion. SUVmax is the highest SUV value of the lesion, and a 40% SUVmax was applied as a threshold to quantitate the MTV. TLG is the product of MTV and mean standardized uptake value (SUVmean) of the area of interest.
The parameter values of DWI and IVIM were calculated using the following equation, where b symbols the diffusion sensitivity, and ADC was computed using two b values (0,1000 s/mm2) fitted to the model. S(b) is the signal strength for different b values, S0 is the signal strength at b = 0 s/mm2, f is the perfusion fraction, D is the diffusion coefficient representing actually molecular diffusion, and D* is the pseudo-diffusion coefficient meaning separate microcirculation [18].
ADC calculation formula [19]:
| 1 |
IVIM calculation formula:
| 2 |
Treatment and follow-up
Of the 61 patients, 32 were treated surgically and 29 were treated non-surgically. Two radiologists (Y L and PY F with 3 years of experience in imaging diagnosis), blined to the clinical and imaging information of patients, collected the follow-up data. The starting time of follow-up was the day of pathological diagnosis (or the day of surgery), and patients were followed up by case retrieval or by telephone until September 30, 2022. All patients were not missed, and the follow-up rate was 100%. Overall survival (OS) was defined as the time from pathological diagnosis to death or the end of follow-up [20]. Progression-free survival (PFS) was defined as the time from pathological diagnosis to the first detection of tumor recurrence, progression, death, or the end of follow-up.
Statistical analysis
Statistical analysis utilized SPSS 20.0, with SUVmax, MTV, TLG, D, f, D*, and ADC categorized as rank information data, while other count data were expressed as percentages. Interobserver reliability between two radiologists was assessed using the intragroup correlation coefficient (ICC), where ICC ≥ 0.75 indicated excellent reliability, 0.60 ≤ ICC < 0.75 indicated good reliability, ICC < 0.40 indicated poor reliability [21]. Survival curves were generated using the Kaplan–Meier method, and group differences were assessed using the Log-rank test. Cox regression analysis was employed for single-factor and multi-factor survival analysis. A significance level of P < 0.05 was applied to all statistical analyses.
Results
Consistency analysis
The parameters measured by the two researchers were in excellent agreement. The ICC values of D, f, D*, ADC were 0.847, 0.956, 0.966, 0.900 respectively. The average value of the two reader parameters is used for subsequent analyses. The values include SUVmax, MTV and TLG were measured by workstation software automatically, and there was no need for a consistency check.
Images and survival
Among the 61 patients, there were 29 males and 32 females with age (62.85 ± 9.63). Moreover, there are 38 patients with disease progression or death, and 23 without progression. Patients had an overall median OS of 18 months (14.75, 22.85) and a median PFS of 17 months (12.00, 21.75). The clinicopathological characteristics are shown in Table 2.
Table 2.
Summary of characteristics
| Characteristics | Value |
|---|---|
| Age (years) | 62.85 ± 9.63 |
| Gender | |
| Male | 29(47.54%) |
| Female | 32(52.46%) |
| Pathological subtype | |
| Adenocarcinoma | 46(75.41%) |
| Squamous carcinoma | 15(24.59%) |
| Maximum diameter (mm) | 27.39 ± 16.78 |
| TNM staging | |
| I ~ II | 24(39.34%) |
| III ~ IV | 37(60.66%) |
| 0peration | |
| yes | 32(52.46%) |
| no | 29(47.54%) |
| EFGR mutation | |
| yes | 10(16.39%) |
| no | 51(83.61%) |
| PFS(month) | 17.00 (12.00, 21.75) |
| OS(month) | 18.00 (14.75, 22.85) |
| PET/MRI-derived Parameters | |
| SUVmax | 5.90(4.38, 10.40) |
| MTV(mL) | 4.82(2.19, 7.91) |
| TLG(g) | 14.32(4.56, 37.61) |
| ADC(× 10–3 mm2 /s) | 1.42(1.27, 1.56) |
| D (× 10−3mm2/second) | 1.20(1.02, 1.30) |
| f (%) | 34.62(24.49, 69.75) |
| D*(× 10−5mm2/second) | 49.68(16.59, 94.33) |
PFS Progression-free survival, OS Overall survival, SUVmax Maximum standard uptake value, MTV Metabolic tumor volume, TLG Total lesion glycolysis, D Diffusion coefficient, f Perfusion fraction, D* Pseudo diffusion coefficient, ADC Apparent diffusion coefficient, EFGR Epidermal Growth Factor Receptor
Univariate and multifactorial analysis of prognostic impact
The results of univariate analysis showed that SUVmax, MTV, TLG, D, D* and ADC were all influential factors for OS and PFS in NSCLC patients among PET/MR-derived metabolic and diffusion parameters (P < 0.05). Among clinical factors, pathological subtype, TNM stage (TNM classification, 8th edition, 2014) and surgery were influential factors for OS and PFS in NSCLC patients (P < 0.05). Factors that were statistically significant in the univariate analysis were introduced as variables in the Cox model for multifactor analysis, and the results showed that MTV, D* and ADC were independent predicting factors for OS and PFS in NSCLC patients (Tables 3, 4; Figs. 1, 2).
Table 3.
Prognostic analysis of OS
| Parameters | univariate analysis | multifactor analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender | 0.399 (0.133—1.197) | 0.101 | / | / |
| Age | 1.037(0.975—1.102) | 0.249 | / | / |
| Diameter | 0.046 (0.000—2820.388) | 0.583 | / | / |
| Pathological subtype | 3.541 (1.239—10.118) | 0.018 | 5.407 (0.794—36.837) | 0.085 |
| TNM staging | 5.372 (1.178—24.491) | 0.030 | 5.189 (0.336—80.143) | 0.238 |
| Operation | 5.393 (1.483—19.610) | 0.011 | 0.420(0.090—1.947) | 0.267 |
| EFGR mutation | 0.846(0.189—3.785) | 0.827 | / | / |
| SUVmax | 1.165 (1.026—1.324) | 0.018 | 1.124 (0.844—1.497) | 0.423 |
| MTV | 1.162 (1.082—1.248) | < 0.001 | 1.216 (1.020—1.450) | 0.029 |
| TLG | 1.036 (1.019—1.053) | < 0.001 | 0.995 (0.943—1.050) | 0.862 |
| ADC | 0.008 (0.000—0.123) | 0.001 | 0 (0.000—0.010) | 0.001 |
| D | 0.124(0.019—0.813) | 0.003 | 2.060(0.003—1546.416) | 0.831 |
| f | 1.001(0.999—1.004) | 0.315 | / | / |
| D* | 0.980(0.963—0.998) | 0.031 | 0.933(0.886—0.982) | 0.008 |
All factors with P < 0.05 in univariate analyses were included in multivariate regression analyses. The bold typeface in the table indicates the logistic regression analyses with statistical significance
EFGR Epidermal growth factor receptor, SUVmax maximum standard uptake value, MTV Metabolic tumor volume, TLG Total lesion glycolysis, D Diffusion coefficient, f Perfusion fraction, D* Pseudo diffusion coefficient, ADC Apparent diffusion coefficient, HR Hazard ratio, CI Confidence interval
Table 4.
Prognostic analysis of PFS
| Parameters | univariate analysis | multifactor analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Gender | 0.453 (0.152—1.353) | 0.156 | / | / |
| Age | 1.034(0.974—1.099) | 0.269 | ||
| Diameter | 0.045 (0.000—958.904) | 0.543 | / | / |
| Pathological subtype | 3.286 (1.152—9.374) | 0.026 | 3.348 (0.576—19.447) | 0.178 |
| TNM staging | 4.600 (1.028—20.573) | 0.046 | 1.910 (0.145—25.072) | 0.622 |
| Operation | 5.105 (1.420—18.354) | 0.013 | 0.747 (0.155—3.605) | 0.717 |
| EFGR mutation | 0.908(0.203—4.059) | 0.899 | / | / |
| SUVmax | 1.155 (1.019—1.309) | 0.024 | 1.138 (0.842—1.537) | 0.401 |
| MTV | 1.135 (1.063—1.213) | < 0.001 | 1.189 (1.005—1.407) | 0.043 |
| TLG | 1.033 (1.016—1.049) | < 0.001 | 0.987 (0.936—1.042) | 0.642 |
| ADC | 0.013 (0.001—0.179) | 0.001 | 0 (0.000—0.012) | 0.002 |
| D | 0.111(0.017—0.728) | 0.022 | 0.685(0.002—277.791) | 0.902 |
| f | 1.001(0.999—1.004) | 0.351 | / | / |
| D* | 0.978(0.961—0.996) | 0.018 | 0.931(0.887—0.978) | 0.004 |
All factors with P < 0.05 in univariate analyses were included in multivariate regression analyses. The bold typeface in the table indicates the logistic regression analyses with statistical significance
EFGR Epidermal growth factor receptor, SUVmax Maximum standard uptake value, MTV Metabolic tumor volume, TLG Total lesion glycolysis, D Diffusion coefficient, f Perfusion fraction, D* Pseudo diffusion coefficient, ADC Apparent diffusion coefficient, HR Hazard ratio, CI Confidence interval
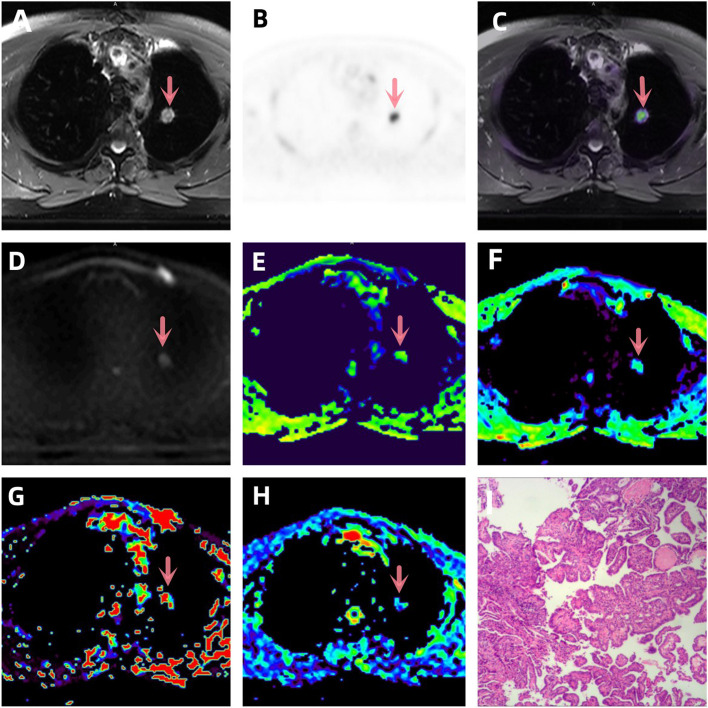
Fig. 1.
A-I a patient with lung adenocarcinoma (arrowheads, stage IVB). A T2WI, B PET original image, SUVmax = 7.72, MTV = 1.207, TLG = 6.033, C PET and T2WI fusion map, D DWI original image (b = 1000 s/mm2), E ADC pseudo-color map, ADC = 2.035 × 10–3 mm2 /s, F D pseudo-color map, D = 1.212 × 10–3 mm2 /s, G D* pseudo-color map, D* = 48.45 × 10–5 mm2 /s, H f pseudo-color map, f = 31.62 × 10–3%, I hematoxylin and eosin (H&E) staining image
Fig. 2.
Kaplan–Meier survival curves of progression-free survival and overall survival stratified by prediction model. a The P-value of log-rank is 0.004; b the P-value of log-rank is 0.005; c the P-value of log-rank is 0.035; d the P-value of log-rank is 0.085; e the P-value of log-rank is 0.032; f the P-value of log-rank is 0.014. 1 means greater than the median, 0 means less than the median
Discussion
Due to the high malignancy, aggressiveness and susceptibility to distant metastasiss of NSCLC, the fatality rate increases year by year [22]. Accurate assessment of the prognosis of NSCLC patients can help to develop better treatment plans and improve the 5-year survival rate. Compared with PET/CT, PET/MR can reduce radiation dose, improve soft tissue contrast, and provide functional information of the lesion, which shows higher value in early prediction of lung cancer treatment effect [23–26].
SUVmax is the most commonly used semi-quantitative PET metabolic parameter in clinical practice. Some studies have explored its value in the prognostic assessment of NSCLC, but no unanimous conclusion has been reached. In the present study, OS and PFS were significantly lower in the SUVmax > 5.90 group than in the SUVmax ≤ 5.90 group, and SUVmax was an influential factor for OS and PFS in NSCLC patients. This is consistent with the findings of Liu et al. [27], however, Erdem et al. [28] and Nawara et al. [29] concluded that SUVmax did not play a role in the survival of NSCLC patients. These inconsistent results may be due to the fact that SUVmax is influenced by multiple factors, such as blood glucose, FDG injection scan time, device model, image reconstruction method, and partial volume effect. On the other hand, SUVmax is a single pixel value, unlike MTV and TLG which can combine multiple factors to reflect more comprehensively the volumetric load and overall metabolic situation of the tumor. MTV and TLG have already been considered as prognostic indicators for a variety of malignancies, such as esophageal cancer [30], laryngeal cancer [31] and nasopharyngeal cancer [32]. This study used univariate Cox regression analysis to show that higher MTV and TLG were significantly associated with worse OS (P < 0.001) and PFS (P < 0.001) in patients with treated NSCLC, and MTV was also an independent predicting factor for PFS and OS in NSCLC patients. Salavati et al. [33] showed that pretreatment tumor volume parameters, including MTV and TLG, had a strong prognostic effect and similar discriminatory power on OS in patients with locally advanced NSCLC by assessing the relationship between each parameter and the outcome of 196 NSCLC patients using Cox regression analysis. The results of Wen et al. [34] also validated that stage I/II NSCLC cases with increased MTV and TLG had a higher risk of side effects and that TLG was associated with an increased risk of death.
ADC is the representative parameter of DWI, which is usually influenced by cell density and can reflect the degree of restricted diffusion of water molecules in the tissue [35]. The parenchymal part of the tumor shows high signal on ADC, which can effectively highlight the lesion. Normally, the more obvious the diffusion restriction of water molecules, the smaller the ADC value [36]. The multifactorial regression analysis in our study showed that ADC was an independent influence on OS and PFS in NSCLC patients. This is similar to the results of previous studies by Ohno et al. [19], in which ADC could be used as a functional indicator to assess the non-surgical outcome of NSCLC patients. This may be due to the fact that tumors with lower ADC values tend to have necrosis, hypoxia, acidification, and poor perfusion, resulting in lower therapeutic sensitivity to radiotherapy and chemotherapy, and therefore a poorer prognosis [37–39].
D is one of the IVIM parameters, which is influenced by cell density and the composition of extracellular matrix, and can reflect the diffusion of water molecules in tissue. This means that when the ratio of nuclei to the cytoplasm in tissues is high, or when the diffusion movement of water molecule is limited, resulting in a small extracellular space, the D value will be decreased [40]. In the IVIM sequence, both D* and f are perfusion-related parameters, however, they represent different features of blood perfusion. D* mainly reflects the average blood flow velocity, while f measures the fraction of blood volume in the capillary network, reflecting the microscopic translation movement related to blood microcirculation [41]. In this study, patients with high D* value and D value group had higher OS than the low value group, and patients with high D* value group had higher PFS than the low value group too. In addition, Cox regression analysis showed that D* was also an independent influencing factor for OS and PFS. This is consistent with the study of Shi C [42] et al. This may be due to the rapid cell proliferation and microvascularity of tumor invasion resulting in decreased D* values. In this study, there was no statistical difference between the high f-value group and the low f-value group. The reason for this result may be different treatment schemes will affect the f-value result. On the other hand, confounding factors, such as the properties of the tissue in the magnetic field, echo time, and relaxation time of the MRI scan, may also greatly affect the f-value [43]. Therefore, the potential of f in evaluating tumor microcirculation changes needs further study.
This study has several limitations. First, the relatively small number of NSCLC cases, due to the prospective nature of the study. It would lead to partial selection bias, and further studies with multiple centers and large samples are needed. Second, the ROIs of DWI and IVIM avoid necrosis, cystic degeneration and vascular areas, which may not be conducive to a comprehensive evaluation of tumor tissue structure. This is due to the large variation in these areas between lesions, which can interfere significantly with the parameter values. Third, the effect of cardiac and macrovascular pulsations on parameter measurements was not considered in this study.
Conclusion
In summary, SUVmax, MTV, TLG, D, D* and ADC derived from 18F-FDG PET/MR imaging are all influential factors in the prognosis of NSCLC patients. Moreover, MTV, D* and ADC are independent predicting factors for poor prognosis of NSCLC patients. These parameters can be jointly applied in clinical practice to predict treatment response and survival of NSCLC patients, and have important value in developing the personalized treatment and improving the survival for patients. In the future, the combination of PET/MR multi-models to predict NSCLC survival may be a new direction.
Acknowledgements
We acknowledge all those who participated in this research. In addition, Han Jiang is greatful for the love received from Wensheng Zheng. I miss you, grandpa.
Abbreviations
- PET/MR
Positron emission tomography-magnetic resonance
- NSCLC
Non-Small cell lung cancer
- SUVmax
Maximum standard uptake value
- MTV
Metabolic tumor volume
- TLG
Total lesion glycolysis
- D
Diffusion coefficient
- f
Perfusion fraction
- D*
Pseudo diffusion coefficient
- ADC
Apparent diffusion coefficient
- DWI
Diffusion-weighted imaging
- IVIM
Intravoxel incoherent motion
- HR
Hazard ratio
- OS
Overall survival time
- PFS
Progression-free survival time
- OSME
Ordered subset maximum expectation iteration method
- MRAC
MR-based attenuation correction
- SUVmean
Mean standardized uptake value
- ICC
Intragroup correlation coefficient
Authors’ contributions
HJ: Data curation, Methodology, Formal analysis, Writing-Original draft preparation. ZL: Methodology, Writing-Reviewing and Editing. NM: Methodology, investigation. YL: Project administration, Investigation. PF: Project administration, Investigation. FF: Funding acquisition, investigation. YY: Project administration, Investigation. JY: Software, Writing-Reviewing and Editing. ZW: Software. All authors reviewed the manuscript. MW: Conceptualization, Funding acquisition, Methodology, Writing-Reviewing and Editing, Validation.
Funding
This work was supported by the Zhengzhou Collaborative Innovation Major Project [grant number 20XTZX05015]; the medical science and technology project of Henan Province [grant number SBGJ202101002]; the Henan provincial science and technology research projects [grant number 212102310689]; and the Henan Province Medical Science and technology public relations plan joint project [grant number LHGJ20210001]. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This retrospective study was approved by the Ethics Committee of Henan Provincial People's Hospital (2021 Lungren Trial No.148), and the requirement for informed consent was obtained. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Han Jiang, Ziqiang Li and Nan Meng contributed equally to this work and should be considered co-first authors.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10(10):1236–71. [DOI] [PubMed] [Google Scholar]
- 3.Im HJ, Pak K, Cheon GJ, et al. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015;42(2):241–51. [DOI] [PubMed] [Google Scholar]
- 4.Shi A, Wang J, Wang Y, Guo G, Fan C, Liu J. Predictive value of multiple metabolic and heterogeneity parameters of 18F-FDG PET/CT for EGFR mutations in non-small cell lung cancer. Ann Nucl Med. 2022;36(4):393–400. 10.1007/s12149-022-01718-8. [DOI] [PubMed] [Google Scholar]
- 5.Shao X, Niu R, Jiang Z, et al. Role of PET/CT in Management of Early Lung Adenocarcinoma. AJR Am J Roentgenol. 2020;214(2):437–45. 10.2214/AJR.19.21585. [DOI] [PubMed] [Google Scholar]
- 6.Tosi D, Pieropan S, Cattoni M, et al. Prognostic Value of 18F-FDG PET/CT Metabolic Parameters in Surgically Treated Stage I Lung Adenocarcinoma Patients. Clin Nucl Med. 2021;46(8):621–6. 10.1097/RLU.0000000000003714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirshahvalad SA, Metser U, Basso Dias A, et al. 18F-FDG PET/MRI in Detection of Pulmonary Malignancies: A Systematic Review and Meta-Analysis. Radiology. 2023;307(2):e221598. 10.1148/radiol.221598. [DOI] [PubMed] [Google Scholar]
- 8.Moran A, Wang Y, Dyer BA, et al. Prognostic Value of Computed Tomography and/or 18F-Fluorodeoxyglucose Positron Emission Tomography Radiomics Features in Locally Advanced Non-small Cell Lung Cancer. Clin Lung Cancer. 2021;22(5):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y, Hosny A, Zeleznik R, et al. Deep Learning Predicts Lung Cancer Treatment Response from Serial Medical Imaging. Clin Cancer Res. 2019;25(11):3266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu LM, Xu JR, Hua J, et al. Can diffusion-weighted imaging be used as a reliable sequence in the detection of malignant pulmonary nodules and masses? Magn Reson Imaging. 2013;31(2):235–46. [DOI] [PubMed] [Google Scholar]
- 11.Tondo F, Saponaro A, Stecco A, Lombardi M, Casadio C, Carriero A. Role of diffusion-weighted imaging in the differential diagnosis of benign and malignant lesions of the chest-mediastinum. Radiol Med. 2011;116(5):720–33. [DOI] [PubMed] [Google Scholar]
- 12.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 13.Bergamino M, Nespodzany A, Baxter LC, et al. Preliminary Assessment of Intravoxel Incoherent Motion Diffusion-Weighted MRI (IVIM-DWI) Metrics in Alzheimer’s Disease. J Magn Reson Imaging. 2020;52(6):1811–26. 10.1002/jmri.27272. [DOI] [PubMed] [Google Scholar]
- 14.Someya Y, Iima M, Imai H, et al. Investigation of breast cancer microstructure and microvasculature from time-dependent DWI and CEST in correlation with histological biomarkers. Sci Rep. 2022;12(1):6523. Published 2022 Apr 20. 10.1038/s41598-022-10081-7 [DOI] [PMC free article] [PubMed]
- 15.Gao S, Du S, Lu Z, et al. Multiparametric PET/MR (PET and MR-IVIM) for the evaluation of early treatment response and prediction of tumor recurrence in patients with locally advanced cervical cancer. Eur Radiol. 2020;30(2):1191–201. 10.1007/s00330-019-06428-w. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Wang X, Cui Y, et al. Comparative Study of Monoexponential, Intravoxel Incoherent Motion, Kurtosis, and IVIM-Kurtosis Models for the Diagnosis and Aggressiveness Assessment of Prostate Cancer. Front Oncol. 2020;10:1763. Published 2020 Sep 11. 10.3389/fonc.2020.01763 [DOI] [PMC free article] [PubMed]
- 17.Fang T, Meng N, Feng P, et al. A Comparative Study of Amide Proton Transfer Weighted Imaging and Intravoxel Incoherent Motion MRI Techniques Versus (18) F-FDG PET to Distinguish Solitary Pulmonary Lesions and Their Subtypes. J Magn Reson Imaging. 2022;55(5):1376–90. 10.1002/jmri.27977. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Luo Y, Jiang H, et al. The value of diffusion kurtosis imaging, diffusion weighted imaging and 18F-FDG PET for differentiating benign and malignant solitary pulmonary lesions and predicting pathological grading. Front Oncol. 2022;12:873669. Published 2022 Jul 29. 10.3389/fonc.2022.873669 [DOI] [PMC free article] [PubMed]
- 19.Ohno Y, Koyama H, Yoshikawa T, et al. Diffusion-weighted MRI versus 18F-FDG PET/CT: performance as predictors of tumor treatment response and patient survival in patients with non-small cell lung cancer receiving chemoradiotherapy. AJR Am J Roentgenol. 2012;198(1):75–82. [DOI] [PubMed] [Google Scholar]
- 20.Ohno Y, Yui M, Yamamoto K, et al. Chemical Exchange Saturation Transfer MRI: Capability for Predicting Therapeutic Effect of Chemoradiotherapy on Non-Small Cell Lung Cancer Patients. J Magn Reson Imaging. 2023;58(1):174–86. [DOI] [PubMed] [Google Scholar]
- 21.Shieh G. Choosing the best index for the average score intraclass correlation coefficient. Behav Res Methods. 2016;48(3):994–1003. [DOI] [PubMed] [Google Scholar]
- 22.Abstracts of Presentations at the Association of Clinical Scientists 143rd Meeting Louisville, KY May 11–14,2022. Ann Clin Lab Sci.2022;52(3):511–525. [PubMed]
- 23.Messerli M, de Galiza Barbosa F, Marcon M, et al. Value of PET/MRI for assessing tumor resectability in NSCLC-intra-individual comparison with PET/CT [published online ahead of print, 2018 Oct 11]. Br J Radiol. 2018;92(1093):20180379. [DOI] [PMC free article] [PubMed]
- 24.Wehrl HF, Sauter AW, Judenhofer MS, et al. Combined PET/MR imaging–technology and applications. Technol Cancer Res Treat. 2010;9(1):5–20. [DOI] [PubMed] [Google Scholar]
- 25.Balyasnikova S, Löfgren J, de Nijs R, et al. PET/MR in oncology: an introduction with focus on MR and future perspectives for hybrid imaging. Am J Nucl Med Mol Imaging. 2012;2(4):458–74. [PMC free article] [PubMed] [Google Scholar]
- 26.Sauter AW, Wehrl HF, Kolb A, et al. Combined PET/MRI: one step further in multimodality imaging. Trends Mol Med. 2010;16(11):508–15. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Wu L, Liu Z, et al. 18F-RGD PET/CT and Systemic Inflammatory Biomarkers Predict Outcomes of Patients With Advanced NSCLC Receiving Combined Antiangiogenic Treatment. Front Oncol.2021;11:671912. Published 2021 Jun 4. [DOI] [PMC free article] [PubMed]
- 28.Erdem V, Selimoğlu Şen H, Kömek H, et al. Prognostic factors in non-small cell lung cancer patients and prognostic importance of PET/CT SUV max value. Tuberk Toraks. 2012;60(3):207–17. [DOI] [PubMed] [Google Scholar]
- 29.Nawara C, Rendl G, Wurstbauer K, et al. The impact of PET and PET/CT on treatment planning and prognosis of patients with NSCLC treated with radiation therapy. Q J Nucl Med Mol Imaging. 2012;56(2):191–201. [PubMed] [Google Scholar]
- 30.Hyun SH, Choi JY, Shim YM, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010;17(1):115–22. [DOI] [PubMed] [Google Scholar]
- 31.Chung MK, Jeong HS, Park SG, et al. Metabolic tumor volume of [18F]-fluorodeoxyglucose positron emission tomography/computed tomography predicts short-term outcome to radiotherapy with or without chemotherapy in pharyngeal cancer. Clin Cancer Res. 2009;15(18):5861–8. [DOI] [PubMed] [Google Scholar]
- 32.Xie P, Yue JB, Zhao HX, et al. Prognostic value of 18F-FDG PET-CT metabolic index for nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2010;136(6):883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salavati A, Duan F, Snyder BS, et al. Optimal FDG PET/CT volumetric parameters for risk stratification in patients with locally advanced non-small cell lung cancer: results from the ACRIN 6668/RTOG 0235 trial. Eur J Nucl Med Mol Imaging. 2017;44(12):1969–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen W, Piao Y, Xu D, et al. Prognostic Value of MTV and TLG of 18F-FDG PET in Patients with Stage I and II Non-Small-Cell Lung Cancer: a Meta-Analysis. Contrast Media Mol Imaging. 2021;2021:7528971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.le Bihan D. Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology. 2013;268(2):318–22. [DOI] [PubMed] [Google Scholar]
- 36.Shen G, Jia Z, Deng H. Apparent diffusion coefficient values of diffusion-weighted imaging for distinguishing focal pulmonary lesions and characterizing the subtype of lung cancer: a meta-analysis. Eur Radiol. 2016;26(2):556–66. [DOI] [PubMed] [Google Scholar]
- 37.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology[J]. AJR Am J Roentgenol. 2007;188(6):1622–35. 10.2214/AJR.06.140. [DOI] [PubMed] [Google Scholar]
- 38.Usuda K, Funasaki A, Sekimura A, et al. FDG-PET/CT and diffusion-weighted imaging for resected lung cancer: correlation of maximum standardized uptake value and apparent diffusion coefficient value with prognostic factors. Med Oncol. 2018;35(5):66. Published 2018 Apr 9. [DOI] [PMC free article] [PubMed]
- 39.Huang YS, Chen JL, Chen JY, et al. Predicting tumor responses and patient survival in chemoradiotherapy-treated patients with non-small-cell lung cancer using dynamic contrast-enhanced integrated magnetic resonance-positron-emission tomography. Vorhersage von Tumoransprechen und Patientenüberleben bei den mit Chemoradiotherapie behandelten Patienten mit nicht-kleinzelligem Lungenkrebs mittels dynamischer kontrastverstärkter integrierter Magnetresonanz-Positronenemissionstomographie. Strahlenther Onkol. 2019;195(8):707–718. [DOI] [PubMed]
- 40.Yuan Z, Niu XM, Liu XM, et al. Use of diffusion-weighted magnetic resonance imaging (DW-MRI) to predict early response to anti-tumor therapy in advanced non-small cell lung cancer (NSCLC): a comparison of intravoxel incoherent motion-derived parameters and apparent diffusion coefficient. Transl Lung Cancer Res. 2021;10(8):3671–81. 10.21037/tlcr-21-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee EY, Yu X, Chu MM, et al. Perfusion and diffusion characteristics of cervical cancer based on intraxovel incoherent motion MR imaging-a pilot study. Eur Radiol. 2014;24(7):1506–13. 10.1007/s00330-014-3160-7. [DOI] [PubMed] [Google Scholar]
- 42.Shi C, Liu D, Xiao Z, et al. Monitoring Tumor Response to Antivascular Therapy Using Non-Contrast Intravoxel Incoherent Motion Diffusion-Weighted MRI. Cancer Res. 2017;77(13):3491–501. 10.1158/0008-5472.CAN-16-2499. [DOI] [PubMed] [Google Scholar]
- 43.Wang LL, Lin J, Liu K, et al. Intravoxel incoherent motion diffusion-weighted MR imaging in differentiation of lung cancer from obstructive lung consolidation: comparison and correlation with pharmacokinetic analysis from dynamic contrast-enhanced MR imaging. Eur Radiol. 2014;24(8):1914–22. 10.1007/s00330-014-3176-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.