Abstract
Background
Cardiovascular disease (CVD) is a major cause of death and recent studies have highlighted the potential role of dietary carbohydrate indices in cardiovascular health. Given the controversial results in this field, the aim of this study was to investigate the association between low carbohydrate dietary score (LCDS) and CVD risk factors in a population of Iranian adults.
Methods
This cross-sectional study was conducted within the framework of the Shiraz Heart Study (SHS) including 1982 adults. The validated 168 items food frequency questionnaire (FFQ) was used to assess participants’ dietary intakes. To investigate the association between LCDS and cardiometabolic risk factors, logistic regression, was conducted.
Results
During 5 years of follow-up, a total of 1982 adults, with a mean age of 53.07 ± 8.38 years, were included to the analysis. The adjusted model based on known confounding factors (age, sex, smoking, physical activity, energy intake and body mass index) revealed a significant decrease in a body shape index (ABSI) (OR = 0.70, 95% CI= (0.50 to 0.98), P = 0.038) comparing highest LCDS tertile vs. the reference. In contrast, risk of hypertension (HTN), body mass index (BMI), waist-hip ratio (WHR), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), atherogenic index of plasma (AIP), and fasting blood sugar (FBS) were not significantly associated with LCDS.
Conclusion
Current findings suggest that adherence to a low carbohydrate diet reduces ABSI, a main indicator of central obesity.
Keywords: Cardiovascular disease, Low carbohydrate diet, Nutrition, Obesity, Cohort study
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, with 422.7 and 17.9 million annual cases and deaths, respectively [1]. CVDs are responsible for one-third of global mortality and one-half of non-communicable diseases (NCDs) [2, 3], while more than three-quarters of CVD mortality was seen in low- to middle-income countries due to poor health facilities and low public awareness [4]. Iran has one of the highest rate of CVD incidence in Asia and around the world, with over 9,000 cases per 100,000 individuals [5]. Dietary choices have a significant impact on the development of obesity, dyslipidemia, hyperglycemia, insulin resistance (IR) and diabetes mellitus (DM), which are the major risk factors for CVD [4, 6–9].
It is crucial to comprehend the association between carbohydrate intake and the prevalence of overweight, obesity and risk factors for CVD, as dietary carbohydrates are acknowledge as the primary energy source [10, 11]. A low-carbohydrate diet (LCD) is defined as a dietary pattern where carbohydrates make up less than 45% of daily energy [11]. Nowadays, carbohydrate-restricted diets have become a popular weight loss strategy. It remains unclear whether LCD can improve biomarkers of cardiovascular health or diseases [11]. However, recent studies have shown that individuals who adhere to a LCD experience greater reductions in weight, total cholesterol (TC) and triglyceride (TG), and increased high density lipoprotein cholesterol (HDL-c) levels, compared to the counterparts on a low-fat diet (LFD) [12–14]. On the other hand, clinical studies contradict the benefits of a LCD and its relationship with metabolic diseases and CVDs [11, 15, 16]. In a cross-sectional study, the results showed that LCD was not associated with CVD risk factors in a group of Iranian women [11]. In addition, some studies have reported that LCD increased LDL-c, fasting glucose, TG levels, and blood pressure (BP) [17–19]. Therefore, given the inconsistent findings in the field, the aim of this study was to investigate the relationship between LCD scores and CVD risk factors in Iranian adults.
Methods
Study design and population
The present cross-sectional study was conducted within the framework of the Shiraz Heart Study (SHS). SHS is a prospective cardiovascular cohort, which planed to be performed in a middle-aged population in Shiraz, Iran, to investigate the CVD risk factors. The present study used the data gathered after 5 years of follow-up.
The details of participant allocation in SHS have been documented elsewhere [20]. Briefly, by allocating 25 clinics and 400 participants for each, the whole sample size of SHS reached 10,000 people, but only 1982 of these participants were eligible to enter the current study. Free-CVD participants who were aged ≥ 30 years at baseline were included if they had lived in the urban areas of Shiraz for at least one year from the date of entering the study and had no plans of migration in the next few years from baseline assessments. Individuals who resided far from the study centers, had mental or physical disabilities, or had a history of coronary artery bypass grafting, percutaneous coronary intervention, positive angiographic reports, invalid dietary data, and a clinical evidence related to CVD outcomes were excluded.
Participants of the study signed informed consent documents. The protocol of the current study was approved by the Ethics Committee of Shiraz University of Medical Sciences (ID: IR.SUMS.SCHEANUT.REC.1401.030).
Data collections and measurements
Dietary intakes and other baseline data, including sociodemographic characteristics (age, gender, smoking, education, physical activity, number of children, dietary intakes), anthropometric indices (body weight, height, body mass index (BMI), waist and hip circumference, waist-hip-ratio (WHR)), laboratory data (such as fasting blood sugar (FBS), blood lipid profile (LDL-c, HDL-c, triglyceride (TG), total cholesterol (TC))), and health status (medications in use, family history of diseases) were collected. The body weight of participants was measured (in the A.M.) using a calibrated digital scale (Sahand, Iran) with an accuracy of 100 g when participants were minimally clothed without shoes. In addition, the participants’ body height was assessed using a stadiometer with an accuracy of 0.1 cm, while standing next to the wall without shoes, and the shoulders, hips, and heels were tangential to the wall, and the head was facing forward. Then, BMI was calculated by dividing the body weight (kg) by the square of the height (m2). The waist circumference (WC) was measured considering the midpoint between the last rib and the iliac crest, and the hip circumference, the largest circumference of the hip was measured by an inflexible meter with an accuracy of 0.1 cm. WHR was obtained by dividing the waist circumference by the hip circumference. A body shape index (ABSI) was calculated through the following formula [21]:
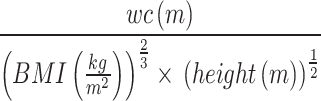 |
Also, the atherogenic index of plasma (AIP) was calculated from the combination of triglyceride and HDL-c indices with the following equation: AIP = log (TG/HDL-c).
Before measuring the blood pressure, the participants were asked to rest for 10 min, then their blood pressure was measured from both arms consecutively using a digital device (OMRON, Germany); and repeated twice with an interval of 10 min. At the end, the mean of three measurements was recorded as the final blood pressure.
Blood samples were collected after 12 h of fasting and analyzed in the laboratory of Shiraz heart center. FBS, TC, and TG were measured by an enzymatic method with an automatic analyzer using kits (Byrex, Iran). HDL-c was determined enzymatically after precipitation of other lipoproteins with dextran magnesium-chloride-sulfate. Friedewald’s equation was used to calculate LDL-c in subjects with TG < 400 mg/dL, however, direct measurement of LDL-c via the turbidity method was performed for subjects with TG ≥ 400 mg/dL.
The International Physical Activity Questionnaire (IPAQ) was used to assess the participants’ physical activities over a week. Questionnaire scores were reported as continuous chains (by METs index) [22].
The validated 168-item food frequency questionnaire (FFQ) was used to assess the participants’ dietary intakes [23]. The amount and frequency of food consumption were evaluated on a daily, weekly, monthly, or yearly basis via an interview with experienced nutritionists at the health center of Shiraz. The amount of each food was converted to grams per day using household standards. The United States Department of Agriculture (USDA) was executed to compute daily energy and dietary components. The Iranian food composition table was used for traditional foods, which were not reported by USDA [24].
LCD score
Seven criteria were considered to calculate the LCDS: the intake amounts of dietary carbohydrate (% total energy intake), fiber (gr/1000 kcal), monounsaturated fatty acids (MUFAs) (% total energy intake), the ratio of omega-3 to omega-6 (n3/n6 PUFA), refined carbohydrate (% total energy intake), vegetable protein (% of total energy intake), and glycemic load (GL) and for each criterion, a score between 0 and 10 was defined.
The participants who had the highest intake in terms of fiber, MUFAs, n3/n6 PUFA, and vegetable proteins scored 10, and those who had the lowest intake scored 0. Regarding the intake of total carbohydrates, refined carbohydrates and GL, participants with the highest intakes scored 0 and those with the lowest intakes scored 10. Then the scores of each item were collected to create a total score that ranged from 0 (lowest consumption of fat and protein and highest consumption of carbohydrates) to 70 (highest consumption of protein and fat and lowest consumption of carbohydrates) [25].
Statistical methods
Data were analyzed using statistical software SPSS version 26 (SPSS Inc., Chicago, IL, USA). Quantitative data were reported as mean (standard deviation (SD)) and qualitative data were reported as frequency (%). The normality of the variables was determined by the Kolmogorov-Smirnov test. To report the amount of daily energy intake and dietary components across tertiles of LCDS and related correlations, we used analysis of variance (ANOVA). To investigate the relationship between LCDS and CVD risk factors, binary logistic regression in a crude and adjusted model, was used. In the adjusted model, the effects of age, gender, smoking, physical activity, daily energy intake, and BMI were controlled, except for BMI and ABSI, which were not adjusted for BMI. Moreover, dietary intakes were adjusted based on 1000 kcal daily energy intake. P-values less than 0.05 were considered significant.
Results
Baseline characteristics
Participants were predominantly female (52.9%), and mean age (SD) was 53.07 (8.38). The baseline characteristics of participants are shown in Table 1. Regarding dietary intakes, compared to the participants in the lowest tertile of LCDS, those in the third tertile had a higher intake of energy, protein, total fat, cholesterol, fiber, dairy products, fruits, and vegetables (P = 0.001). However, lower intakes of total carbohydrates, whole grains, sweets, and refined carbohydrates were seen in the higher tertiles of LCDS (P = 0.001) (Table 2).
Table 1.
Baseline characteristics of the study population
| Characteristic | Total* (n = 1982) |
|---|---|
| Age (year) | 53.0 ± 8.3 |
| Female (%) | 1045 (52.9%) |
| Current smoker (%) | 446 (22.6%) |
|
Physical activity (Low/moderate/high) |
534 (50.3%) 263 (24.8%) 264 (24.9%) |
| SBP (mmHg) | 123 ± 16 |
| DBP (mmHg) | 80 ± 10 |
| Weight (kg) | 74.4 ± 13.4 |
| BMI (Kg/m2) | 27.8 ± 4.6 |
| WC (cm) | 98.4 ± 11.1 |
| Hip Circumference (cm) | 104 ± 8.7 |
| WHR (cm2) | 0.9 ± 0.6 |
| ABSI | 0.08 ± 0.0 |
| TG (mg/dl) | 147 ± 72.7 |
| TC (mg/dl) | 189 ± 40.6 |
| LDL-c (mg/dl) | 108 ± 28.0 |
| HDL-c (mg/dl) | 47.8 ± 10.5 |
| AIP | 0.4 ± 0.2 |
| FBS (mg/dl) | 104 ± 28.8 |
*Data are expressed as mean ± SD for continuous or frequency (percent) for categorical variables; Abbreviations: (HTN: Hypertension), (BMI: Body mass index), (WHR: Waist-hip-ratio), (ABSI: A body shape index), (TG: Triglyceride), (TC: Total cholesterol), (LDL-c: Low-density-lipoprotein cholesterol), (HDL-c: High-density-lipoprotein cholesterol), (AIP: Atherogenic index of plasma), (FBS: Fasting blood sugar)
Table 2.
Baseline dietary intakes of the participants across tertiles of low carbohydrate diet score (LCDS)
| Dietary intakes* | Total (n = 1982) | Low carbohydrate diet score (LCDS) | P # | ||
|---|---|---|---|---|---|
| T1 (n = 661) |
T2 (n = 661) |
T3 (n = 660) |
|||
| Energy (kcal) | 2838 ± 1065 | 2640 ± 1047 | 2809 ± 971 | 3064 ± 1123 | 0.001 |
| Carbohydrate (g/d) | 159 ± 16.9 | 169 ± 12.5 | 158 ± 15.8 | 149 ± 15.7 | 0.001 |
| Protein (g/d) | 34.6 ± 5.9 | 31.3 ± 4.2 | 34.5 ± 5.0 | 38.0 ± 6.3 | 0.001 |
| Fat (g/d) | 28.2 ± 7.5 | 24.5 ± 5.5 | 28.7 ± 7.6 | 31.3 ± 7.7 | 0.001 |
| Dietary fibers (g/d) | 16.1 ± 4.8 | 14.0 ± 3.3 | 16.0 ± 4.4 | 18.3 ± 5.4 | 0.001 |
| Cholesterols (g/d) | 76.8 ± 32.8 | 68.6 ± 30.1 | 77.6 ± 29.7 | 84.3 ± 36.1 | 0.001 |
| Whole grains (g/d) | 37.4 ± 33.5 | 38.1 ± 38.3 | 40.5 ± 34.6 | 33.9 ± 26.3 | 0.001 |
| Fruits (g/d) | 214 ± 109 | 198 ± 106 | 221 ± 110 | 223 ± 108 | 0.001 |
| Vegetables (g/d) | 190 ± 93.7 | 166 ± 76.9 | 189 ± 87.9 | 215 ± 107 | 0.001 |
| Refined grains (g/d) | 111 ± 52.7 | 149 ± 54.2 | 105 ± 40.0 | 77.7 ± 32.8 | 0.001 |
| Dairy (g/d) | 98.7 ± 70.6 | 83.5 ± 63.2 | 100 ± 68.7 | 112.7 ± 76.3 | 0.001 |
| Sweets (g/d) | 46.2 ± 44.0 | 57.2 ± 53.2 | 43.8 ± 39.4 | 37.2 ± 34.6 | 0.001 |
* Total dietary intakes were adjusted based on 1000 Kcal intake of daily energy; data are expressed as mean ± SD;
# P-values less than 0.05 are considered significant and one-way ANOVA was used for the analysis
Association of LCDS with CVD risk factors
The association of LCDS with the CVD risk factors is shown in Table 3. Higher LCDS was associated with higher BMI and WHR in the crude model in the participants of the third tertile in comparison with the reference ones. However, after adjusting for confounders (age, gender, smoking, physical activity, daily energy intake and BMI, except for BMI and ABSI, which were not adjusted for BMI), the significant associations were attenuated. The adjusted model, based on known confounders, revealed a significant reduction in ABSI (OR = 0.70, 95% CI= [0.50 to 0.98]) following higher LCDS vs the reference. In contrast, HTN (OR = 0.94, 95% CI=[0.68 to 1.31]), BMI (OR = 1.01, 95% CI=[0.71 to 1.43]), WHR (OR = 0.84, 95% CI=[0.50 to 1.40]), TG (OR = 0.82, 95% CI=[0.59 to 1.15]), TC (OR = 0.99, 95% CI=[0.71 to 1.38]), LDL-c (OR = 0.88, 95% CI=[0.59 to 1.20]), HDL-c (OR = 0.92, 95% CI=[0.67 to 1.27]), AIP (OR = 1.16, 95% CI=[0.83 to 1.62]) and FBS (OR = 0.87, 95% CI=[0.52 to 1.45]) were not significantly associated with LCDS when comparing the 3rd tertile vs 1st one.
Table 3.
Odds ratio of cardiometabolic risk factors according to LCDS tertile
| Models | T1 (n = 661) | T2 (n = 661) | T3 (n = 660) | P # |
|---|---|---|---|---|
| HTN (mmHg) | ||||
|
Crude Adjusted* |
1.00 1.00 |
0.97 (0.77–1.21) 0.91 (0.66–1.26) |
0.87 (0.70–1.09) 0.23 0.94 (0.68–1.31) 0.57 |
0.23 0.57 |
| BMI (kg/m2) | ||||
|
Crude Adjusted |
1.00 1.00 |
1.34 (1.05–1.71) 1.18 (0.84–1.68) |
1.48 (1.16–1.89) <0.001 1.01 (0.71–1.43) 0.94 |
<0.001 0.94 |
| WHR | ||||
|
Crude Adjusted |
1.00 1.00 |
1.50 (1.17–1.94) 0.81 (0.50–1.30) |
1.78 (1.38–2.31) <0.001 0.84 (0.50–1.40) 0.51 |
<0.001 0.51 |
| ABSI | ||||
|
Crude Adjusted |
1.00 1.00 |
0.88 (0.71–1.11) 0.82 (0.59–1.13) |
0.82 (0.66–1.03) 0.09 0.70 (0.50–0.98) 0.03 |
0.09 0.03 |
| TG (mg/dl) | ||||
|
Crude Adjusted |
1.00 1.00 |
0.97 (0.77–1.21) 0.89 (0.64–1.23) |
0.94 (0.75–1.18) 0.64 0.82 (0.59–1.15) 0.26 |
0.64 0.26 |
| TC (mg/dl) | ||||
|
Crude Adjusted |
1.00 1.00 |
1.05 (0.84–1.32) 1.08 (0.78–1.49) |
1.02 (0.81–1.27) 0.86 0.99 (0.71–1.38) 0.99 |
0.86 0.99 |
| LDL-c (mg/dl) | ||||
|
Crude Adjusted |
1.00 1.00 |
1.04 (0.80–1.37) 0.95 (0.65–1.39) |
1.10 (0.85–1.44) 0.44 0.88 (0.59–1.30) 0.52 |
0.44 0.52 |
| HDL-c (mg/dl) | ||||
|
Crude Adjusted |
1.00 1.00 |
0.99 (0.79–1.24) 1.20 (0.88–1.65) |
0.98 (0.79–1.22) 0.91 0.92 (0.67–1.27) 0.63 |
0.91 0.63 |
| AIP | ||||
|
Crude Adjusted |
1.00 1.00 |
1.11 (0.88–1.39) 0.97 (0.70–1.35) |
1.23 (0.98–1.54) 0.06 1.16 (0.83–1.62) 0.36 |
0.06 0.36 |
| FBS (mg/dl) | ||||
|
Crude Adjusted |
1.00 1.00 |
0.93 (0.64–1.34) 1.02 (0.60–1.71) |
0.81 (0.57–1.15) 0.23 0.87 (0.52–1.45) 0.59 |
0.23 0.59 |
Abbreviations: (HTN: Hypertension), (BMI: Body mass index), (WHR: Waist-hip-ratio), (ABSI: A body shape index), (TG: Triglyceride), (TC: Total cholesterol), (LDL-c: Low-density-lipoprotein cholesterol), (HDL-c: High-density-lipoprotein cholesterol), (AIP: Atherogenic index of plasma), (FBS: Fasting blood sugar)
*Adjusted for of age, gender, smoking, physical activity, daily energy intakes and BMI (except for BMI and ABSI that are not adjusted for BMI)
# P < 0.05 was considered statistically significant (Obtained from logistic regression)
Results are defined as odds ratio (OR) and 95% confidence interval (CI)
Discussion
In the present cross-sectional study, we found an inverse association between LCDS and ABSI independent from known confounders (age, gender, smoking, physical activity, and daily energy intake). In contrast, the HTN, FBS, AIP, lipid profiles, BMI, WC, and WHR were not found to be associated with higher LCDS, which is in accordance with Jafari-Maram et al., who reported a lack of association between low carbohydrate diet and cardiovascular risk factors [11].
Mirmiran and colleagues [25] found an inverse association between metabolic syndrome components and higher LCDS during 3.6 years of follow-up, which was divergent from the results of the current study regarding the association between cardiovascular risk factors and LCDS, which could be due to the difference in the study design. In a review assessing RCTs, the authors concluded that low carbohydrate diets may improve cardiovascular risk factors, accelerate weight loss, and even recommended a low carbohydrate diet as an appropriate strategy for weight management and cardiovascular protection through improving the carbohydrate quality and quantity [26]. Moreover, in a meta-analysis evaluating the effects of low carbohydrate diets in RCTs, Dong et al. found that these diets could possibly improve cardiovascular risk factors in study durations of shorter than 6 months, while for confirming the beneficial effects in the longer-term, further studies are warranted [27]. One of the main reasons for such a different findings could pertain to the fact that the aforementioned reviews assessed the results of the RCTs that could better demonstrate the causal relationships compared to the cross-sectional studies.
Previous studies including cohort and RCTs have shown controversial results regarding the relationship between body weight and waist circumference with low carbohydrate diet [26, 28–32]. In the present study, low carbohydrate diet was associated with lower ABSI. ABSI emphasizes on the distribution of fat, especially the abdominal fat, by considering WC. A higher BMI could either reveal a higher amount of lean mass or fat mass and unlikely to be a good marker of central obesity [21]. ABSI included both WC and BMI,that could be considered as a marker of fat distribution and fat accumulation [21, 33]. Even it was asserted that ABSI could be a better predictor of diabetes and all-cause mortality than BMI in some populations [33, 34], a meta-analysis concluded that it underperformed BMI and WC in predicting chronic diseases [35]. A recent study shed light on the clinical performance of ABSI in identifying the presence of metabolic syndrome and arterial stiffness [36].
It was previously reported that low carbohydrate diets could decrease the odds of central obesity, especially through decreasing fat mass [26, 37]. Moreover, it has been asserted that low carbohydrate diets could decrease waist circumference, to an even greater extent than low-fat diets [38–40]. Hence, the inverse association between LCDS and ABSI could likely confirm the protective effects of low carbohydrate diets on decreasing the risk of central obesity and high ABSI. Possibly, this could indirectly show the protective effects of low carbohydrate diets against central obesity and metabolic syndrome due to the correlations between ABSI and obesity [41] as it was mentioned previously. However, this issue needs further investigation to achieve more definite results.
Further, in the current study, those in the higher tertiles of LCDS had higher dietary intakes of fruits, vegetables, fiber, and dairy products, and lower intakes of total carbohydrates, sweets, and refined carbohydrates, which can, possibly, beneficially affect WC and ABSI. This can partially justify the inverse association between ABSI and LCDS. Similar dietary intakes including lower consumption of total and refined carbohydrate and whole grains and higher intakes of dairy products was also reported in those with higher LCDS in a study by Jafari-maram and colleagues in an Iranian population [11]. Shirani et al. also reported lower intakes of total and refined carbohydrates in those with LCDS [15]. Hence, a lower carbohydrate intake was accompanied by higher dietary fiber, dairy products and lower refined sugar in the present study, which could be associated with improvements in ABSI. This finding was also confirmed by the studies reporting the decreasing effects of fiber [42] and dairy products [43] on WC.
There are several limitations to the interpretation of our findings as well. Of note, the cross-sectional design did not allow for the establishment of a causal relationship, only associations between variables. Although we attempted to control for potential confounders, residual confounding could remain because of unmeasured confounders. As the Iranian food composition table was incomplete, we mostly used the USDA nutrient databank for the dietary analyses. In addition, due to the differences in the food culture and dietary habits, it will be difficult to generalize our findings to other societies. However, the main strength of our study lies in the relatively large sample size of SHS. In addition, we used a validated FFQ for Iranian and measured variables in regard to the standard methods. Enrolled participants were free from CVD at baseline, which decreases the risk of diet modification in response to disease.
Conclusion
Adherence to a low carbohydrate diet was not associated with main CVD risk factors in an Iranian population. So far, a low carbohydrate diet may be able to decrease ABSI, as an indicator of central obesity that includes WC as a main component in its calculation. A low carbohydrate diet in this study was accompanied by higher dietary intakes of fruits, vegetables, fiber, and dairy products, and lower intakes of total carbohydrates, sweets, and refined carbohydrates that could improve ABSI. Further investigations in different populations and with various study designs are needed to better confirm these results.
Acknowledgements
We would like to thank all the members of the Shiraz Heart Study (SHS) for their cooperation and all the study participants that took part in this study. This study was extracted from the thesis by Zahra Mosallanezhad and supported by Shiraz University of Medical Sciences, Shiraz, Iran.
Abbreviations
- CVD
Cardiovascular disease
- LCDS
low carbohydrate dietary score
- SHS
Shiraz Heart Study
- FFQ
Food frequency questionnaire
- NCD
non-communicable disease
- IR
Insulin resistance
- DM
diabetes mellitus
- HDl-c
high-density lipoprotein cholesterol
- LDL-c
low-density lipoprotein cholesterol
- TG
triglyceride
- TC
total cholesterol
- LFD
low fat diet
- BP
blood pressure
- BMI
body mass index
- WHR
waist-hip ratio
- FBS
fasting blood sugar
- WC
waist circumference
- ABSI
a body shape index
- AIP
atherogenic index of plasma
- IPAQ
International physical activity questionnaire
- USDA
the United States Department of Agriculture
- MUFA
monounsaturated fatty acid
- PUFA
polyunsaturated fatty acid
- GL
glycemic load
- GI
glycemic index
- SD
standard deviation
- HTN
hypertension
- RCT
randomized controlled trial
Author contributions
ZM and ZS conceptualized the study. MJZ, SSM, MS, IRJ, NP and FZ developed the methodology. MJ, ZM, CC and ZS wrote the original draft. MJ, MJZ and MN reviewed and edited the manuscript. ZS and MJZ supervised and administered the project and acquired funding. All of the authors contributed significantly to the work and accepted the final manuscript to be published.
Funding
This study was supported by Shiraz University of Medical Sciences (Grant number: 25589).
Data availability
Data will be presented upon forwarding the request to the corresponding author (zahra_2043@yahoo.com) and confirmation of the director of SHS (zibaeem2@gmail.com).
Declarations
Ethics approval and consent to participate
We obtained written informed consent from all participants. Based on the ethical guidelines of the 1975 Declaration of Helsinki, the study protocol was approved by research Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran IR.SUMS.SCHEANUT.REC.1401.030).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roth GA, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laslett LJ, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60(25S):S1–49. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffari H, et al. Associations between dietary insulin load with cardiovascular risk factors and inflammatory parameters in elderly men: a cross-sectional study. Br J Nutr. 2019;121(7):773–81. [DOI] [PubMed] [Google Scholar]
- 4.Emamian MH, Hashemi H, Fotouhi A. Predicted 10-year risk of cardiovascular disease in the Islamic Republic of Iran and the body mass index paradox. East Mediterr Health J, 2020. 26(12). [DOI] [PubMed]
- 5.Sarrafzadegan N, Mohammmadifard N. Cardiovascular Disease in Iran in the last 40 years: Prevalence, Mortality, Morbidity, challenges and Strategies for Cardiovascular Prevention. Arch Iran Med. 2019;22(4):204–10. [PubMed] [Google Scholar]
- 6.Patel H, et al. Plant-based nutrition: an essential component of cardiovascular disease prevention and management. Curr Cardiol Rep. 2017;19(10):1–10. [DOI] [PubMed] [Google Scholar]
- 7.Casas R, et al. Nutrition and cardiovascular health. Int J Mol Sci. 2018;19(12):3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayadi M, Zibaeenezhad MJ, Ayatollahi SMT. Simple prediction of type 2 diabetes mellitus via decision tree modeling. Int Cardiovasc Res J, 2017. 11(2).
- 9.Zargaran ZH, et al. The effects of 6 isocaloric meals on body weight, lipid profiles, leptin, and adiponectin in overweight subjects (BMI > 25). Int Cardiovasc Res J. 2014;8(2):52. [PMC free article] [PubMed] [Google Scholar]
- 10.Zazpe I, et al. Association between a dietary carbohydrate index and cardiovascular disease in the SUN (Seguimiento Universidad De Navarra) Project. Nutr Metabolism Cardiovasc Dis. 2016;26(11):1048–56. [DOI] [PubMed] [Google Scholar]
- 11.Jafari-Maram S, et al. Association of low-carbohydrate diet score with overweight, obesity and cardiovascular disease risk factors: a cross-sectional study in Iranian women. J Cardiovasc Thorac Res. 2019;11(3):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansoor N, et al. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115(3):466–79. [DOI] [PubMed] [Google Scholar]
- 13.Samaha FF, Foster GD, Makris AP. Low-carbohydrate diets, obesity, and metabolic risk factors for cardiovascular disease. Curr Atheroscler Rep. 2007;9(6):441–7. [DOI] [PubMed] [Google Scholar]
- 14.Hu T, et al. The effects of a low-carbohydrate Diet vs. a low-Fat Diet on Novel Cardiovascular Risk factors: a Randomized Controlled Trial. Nutrients. 2015;7(9):7978–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shirani F, et al. Low-carbohydrate-diet score and metabolic syndrome: an epidemiologic study among Iranian women. Nutrition. 2015;31(9):1124–30. [DOI] [PubMed] [Google Scholar]
- 16.Gardner CD, et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319(7):667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalam F, et al. Alternate day fasting combined with a low-carbohydrate diet for weight loss, weight maintenance, and metabolic disease risk reduction. Volume 5. Obesity Science & Practice; 2019. pp. 531–9. 6. [DOI] [PMC free article] [PubMed]
- 18.Perissiou M, et al. The effect of an 8 week prescribed exercise and low-carbohydrate diet on cardiorespiratory fitness, body composition and cardiometabolic risk factors in obese individuals: a randomised controlled trial. Nutrients. 2020;12(2):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gjuladin-Hellon T, et al. Effects of carbohydrate-restricted diets on low-density lipoprotein cholesterol levels in overweight and obese adults: a systematic review and meta-analysis. Nutr Rev. 2019;77(3):161–80. [DOI] [PubMed] [Google Scholar]
- 20.Zibaeenezhad MJ, et al. Analysing cardiovascular risk factors and related outcomes in a middle-aged to older adults population in Iran: a cohort protocol of the shiraz heart study (SHS). BMJ open. 2019;9(4):e026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7(7):e39504. [DOI] [PMC free article] [PubMed]
- 22.Moghaddam MB, et al. The Iranian version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J. 2012;18(8):1073–80. [Google Scholar]
- 23.Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. 2010;13(5):654–62. [DOI] [PubMed]
- 24.Farjam M, et al. A cohort study protocol to analyze the predisposing factors to common chronic non-communicable diseases in rural areas: Fasa Cohort Study. BMC Public Health. 2016;16(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirmiran P, et al. Low carbohydrate diet is associated with reduced risk of metabolic syndrome in tehranian adults. Int J Food Sci Nutr. 2017;68(3):358–65. [DOI] [PubMed] [Google Scholar]
- 26.Hu T, Bazzano L. The low-carbohydrate diet and cardiovascular risk factors: evidence from epidemiologic studies. Nutr Metabolism Cardiovasc Dis. 2014;24(4):337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong T, et al. The effects of low-carbohydrate diets on cardiovascular risk factors: a meta-analysis. PLoS ONE. 2020;15(1):e0225348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster GD, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–90. [DOI] [PubMed] [Google Scholar]
- 29.Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344. [DOI] [PMC free article] [PubMed]
- 30.Nilsson LM, et al. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur J Clin Nutr. 2012;66(6):694–700. [DOI] [PubMed] [Google Scholar]
- 31.Lagiou P, et al. Low carbohydrate–high protein diet and mortality in a cohort of Swedish women. J Intern Med. 2007;261(4):366–74. [DOI] [PubMed] [Google Scholar]
- 32.Trichopoulou A, et al. Low-carbohydrate–high-protein diet and long-term survival in a general population cohort. Eur J Clin Nutr. 2007;61(5):575–81. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Q, et al. Capacity of a body shape index and body roundness index to identify diabetes mellitus in Han Chinese people in Northeast China: a cross-sectional study. Diabet Med. 2018;35(11):1580–7. [DOI] [PubMed] [Google Scholar]
- 34.Yang N, Zhuo J, Xie S, Qu Z, Li W, Li Z, et al. A body shape index and its changes in relation to all-cause mortality among the Chinese elderly: a retrospective cohort study. Nutrients. 2023;15(13):2943. [DOI] [PMC free article] [PubMed]
- 35.Ji M, Zhang S, An R. Effectiveness of A Body Shape Index (ABSI) in predicting chronic diseases and mortality: a systematic review and meta‐analysis. Obesity Rev. 2018;19(5):737–759. [DOI] [PubMed]
- 36.Sugiura T, et al. A body shape index could serve to identify individuals with metabolic syndrome and increased arterial stiffness in the middle-aged population. Clin Nutr ESPEN. 2021;46:251–8. [DOI] [PubMed] [Google Scholar]
- 37.Brinkworth GD, et al. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr. 2009;90(1):23–32. [DOI] [PubMed] [Google Scholar]
- 38.Shai I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–41. [DOI] [PubMed] [Google Scholar]
- 39.Dansinger ML, et al. Comparison of the Atkins, Ornish, Weight watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293(1):43–53. [DOI] [PubMed] [Google Scholar]
- 40.Frisch S, et al. A randomized controlled trial on the efficacy of carbohydrate-reduced or fat-reduced diets in patients attending a telemedically guided weight loss program. Cardiovasc Diabetol. 2009;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gazarova M, Galsneiderova M, Meciarova L. Obesity diagnosis and mortality risk based on a body shape index (ABSI) and other indices and anthropometric parameters in university students. Rocz Panstw Zakl Hig. 2019;70(3). [DOI] [PubMed]
- 42.Du H, et al. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am J Clin Nutr. 2010;91(2):329–36. [DOI] [PubMed] [Google Scholar]
- 43.Vergnaud A-C, et al. Dairy consumption and 6-y changes in body weight and waist circumference in middle-aged French adults. Am J Clin Nutr. 2008;88(5):1248–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be presented upon forwarding the request to the corresponding author (zahra_2043@yahoo.com) and confirmation of the director of SHS (zibaeem2@gmail.com).


