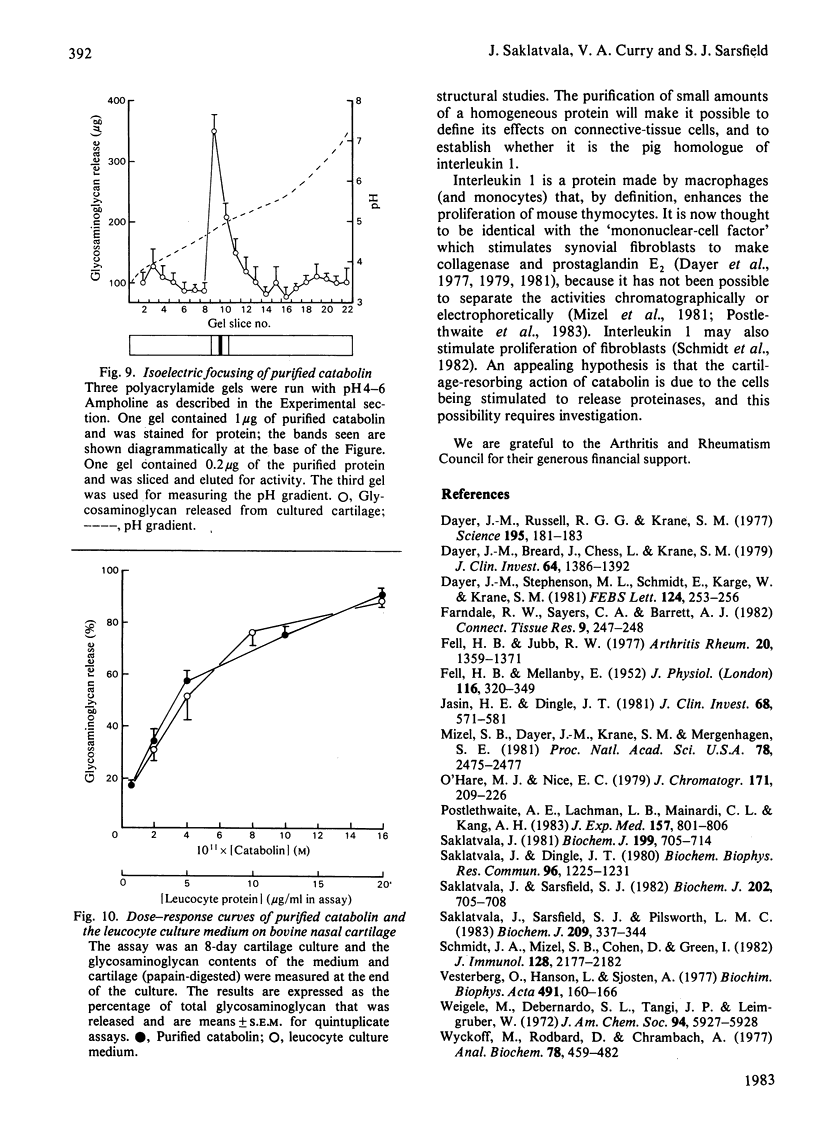
Abstract
Catabolin, a protein that causes proteoglycan resorption in explants of living cartilage, was purified to homogeneity from culture medium conditioned by culturing buffy-coat leucocytes from 60 litres of pig blood in the presence of concanavalin A. The purification steps were (1) gel filtration of concentrated medium, (2) chromatofocusing, (3) hydroxyapatite chromatography, (4) anion-exchange chromatography (Mono Q), (5) reversed-phase high-pressure liquid chromatography (h.p.l.c.) (Zorbax ODS). These achieved approx. 9000-fold purification from the starting material. The purified protein when reduced ran as a single band on sodium dodecyl sulphate (SDS)/polyacrylamide-gel electrophoresis with Mr 21000. On isoelectric focusing its pI was 4.8-5.0, and there was evidence of micro-heterogeneity. The protein co-migrated with active material on h.p.l.c., isoelectric focusing and SDS gels (15 and 12.5% acrylamide) under both reducing and non-reducing conditions. The pure protein caused proteoglycan release from cultured bovine nasal cartilage at 20pM. Its possible identity with interleukin 1 is discussed.
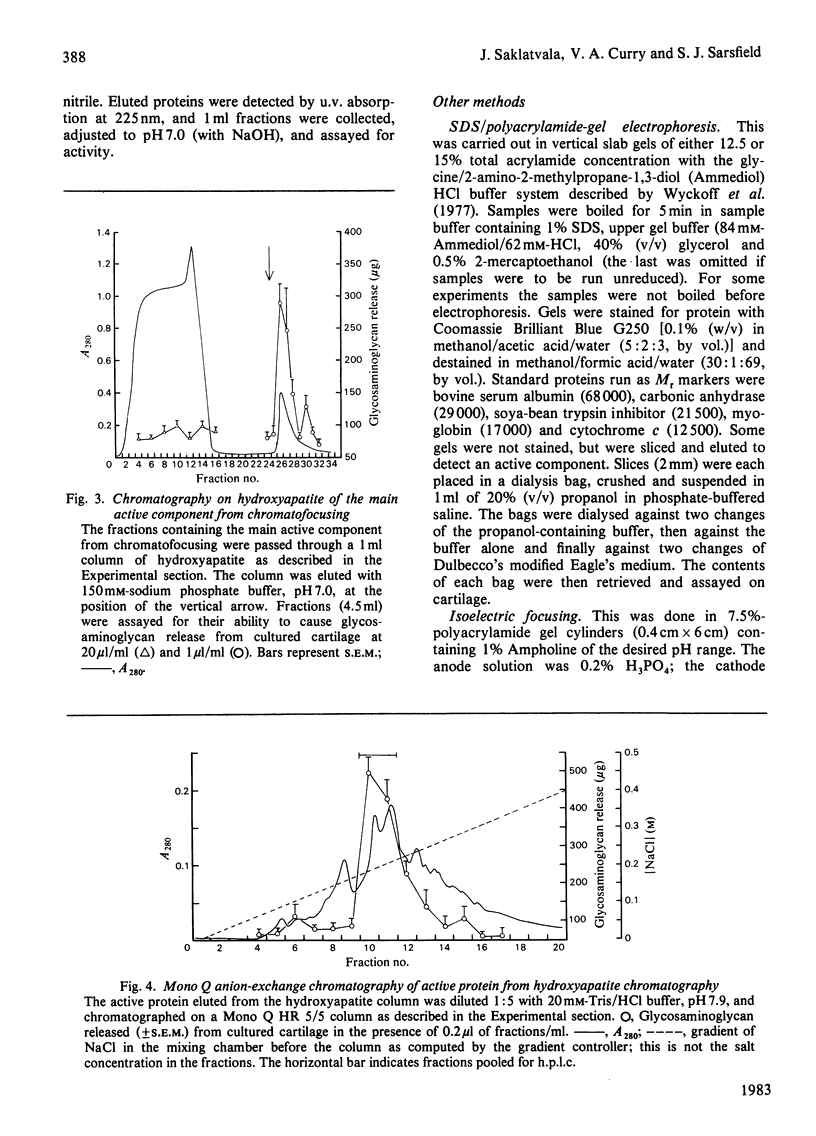
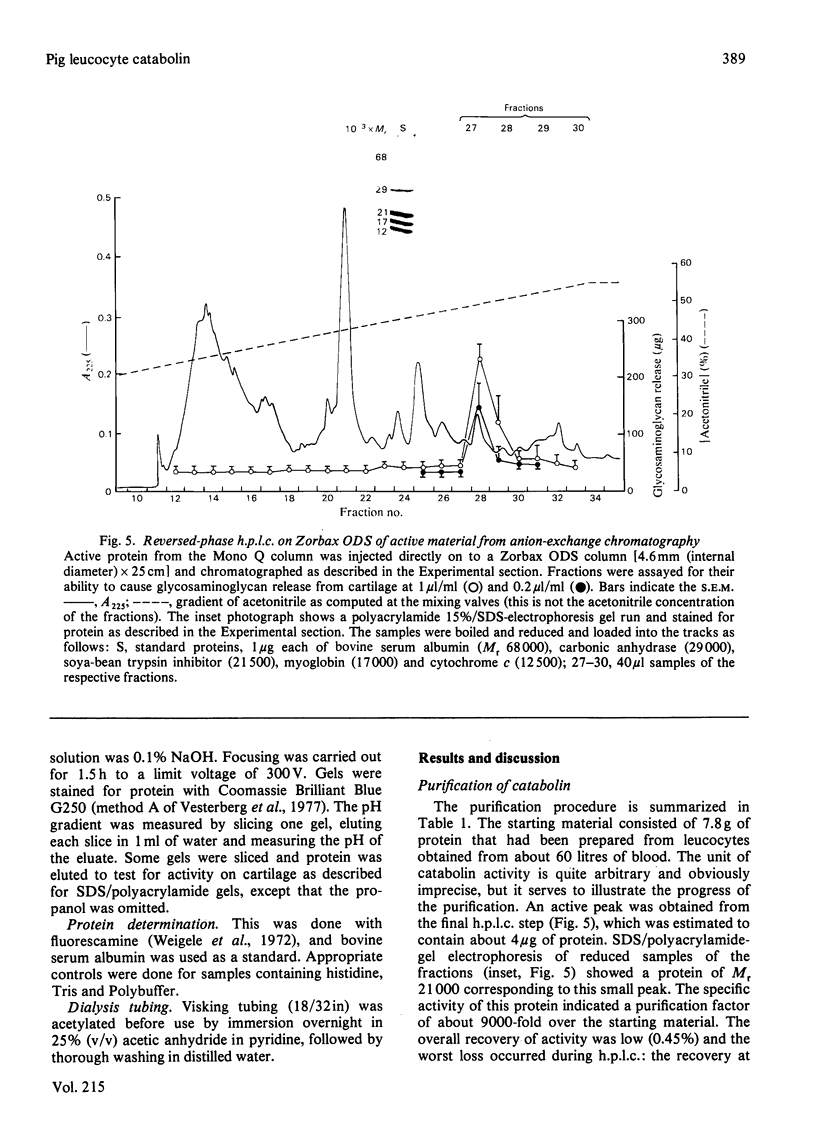
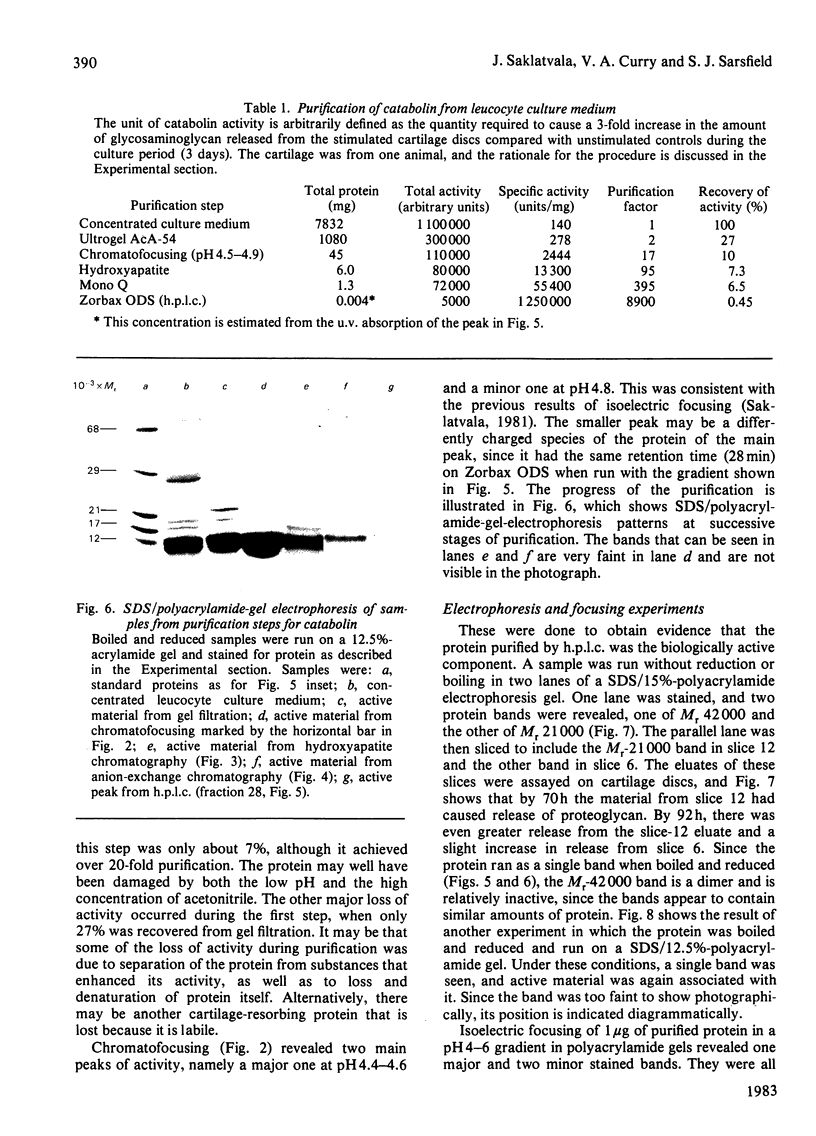
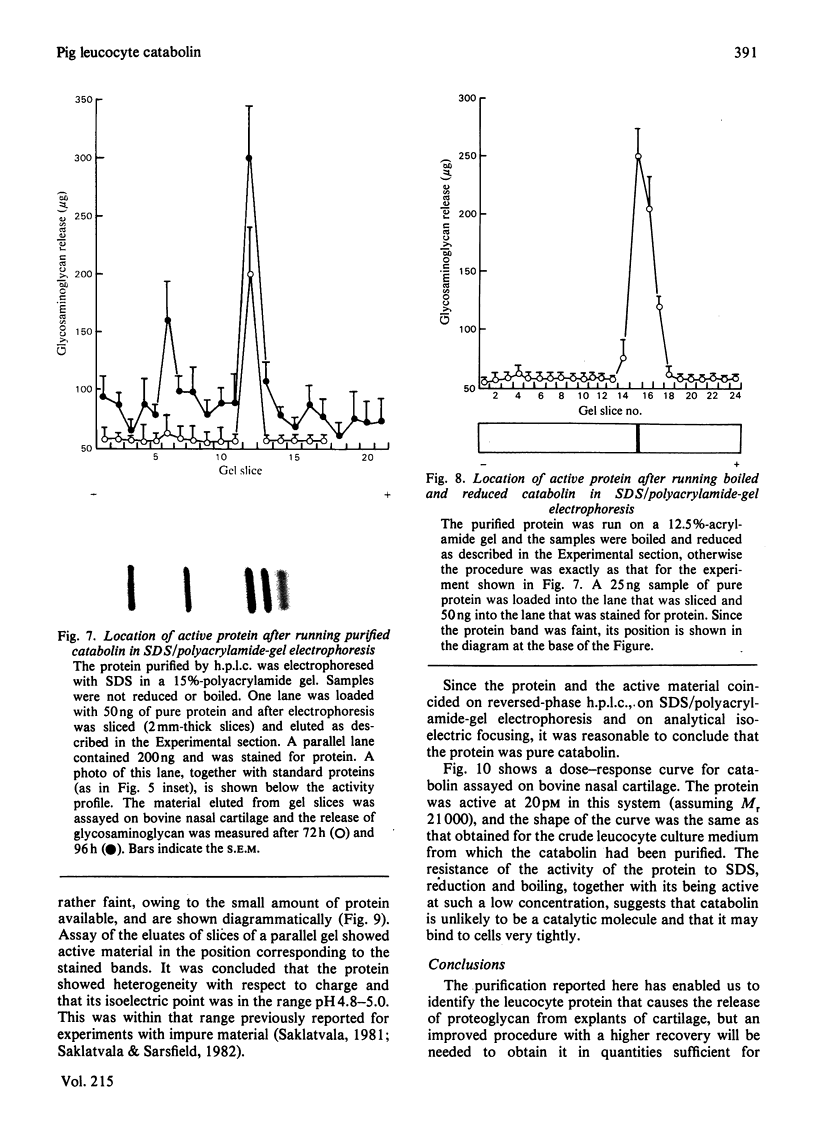
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dayer J. M., Bréard J., Chess L., Krane S. M. Participation of monocyte-macrophages and lymphocytes in the production of a factor that stimulates collagenase and prostaglandin release by rheumatoid synovial cells. J Clin Invest. 1979 Nov;64(5):1386–1392. doi: 10.1172/JCI109596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Graham R., Russell G., Krane S. M. Collagenase production by rheumatoid synovial cells: stimulation by a human lymphocyte factor. Science. 1977 Jan 14;195(4274):181–183. doi: 10.1126/science.188134. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Stephenson M. L., Schmidt E., Karge W., Krane S. M. Purification of a factor from human blood monocyte-macrophages which stimulates the production of collagenase and prostaglandin E2 by cells cultured from rheumatoid synovial tissues. FEBS Lett. 1981 Feb 23;124(2):253–253. doi: 10.1016/0014-5793(81)80149-4. [DOI] [PubMed] [Google Scholar]
- FELL H. B., MELLANBY E. The effect of hypervitaminosis A on embryonic limb bones cultivated in vitro. J Physiol. 1952 Mar;116(3):320–349. doi: 10.1113/jphysiol.1952.sp004708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Fell H. B., Jubb R. W. The effect of synovial tissue on the breakdown of articular cartilage in organ culture. Arthritis Rheum. 1977 Sep-Oct;20(7):1359–1371. doi: 10.1002/art.1780200710. [DOI] [PubMed] [Google Scholar]
- Jasin H. E., Dingle J. T. Human mononuclear cell factors mediate cartilage matrix degradation through chondrocyte activation. J Clin Invest. 1981 Sep;68(3):571–581. doi: 10.1172/JCI110290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare M. J., Nice E. C. Hydrophobic high-performance liquid chromatography of hormonal polypeptides and proteins on alkylsilane-bonded silica. J Chromatogr. 1979 Apr 1;171:209–226. doi: 10.1016/s0021-9673(01)95300-2. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Lachman L. B., Mainardi C. L., Kang A. H. Interleukin 1 stimulation of collagenase production by cultured fibroblasts. J Exp Med. 1983 Feb 1;157(2):801–806. doi: 10.1084/jem.157.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J. Characterization of catabolin, the major product of pig synovial tissue that induces resorption of cartilage proteoglycan in vitro. Biochem J. 1981 Dec 1;199(3):705–714. doi: 10.1042/bj1990705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Dingle J. T. Identification of catabolin, a protein fro synovium which induces degradation of cartilage in organ culture. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1225–1231. doi: 10.1016/0006-291x(80)90082-0. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Sarsfield S. J., Pilsworth L. M. Characterization of proteins from human synovium and mononuclear leucocytes that induce resorption of cartilage proteoglycan in vitro. Biochem J. 1983 Feb 1;209(2):337–344. doi: 10.1042/bj2090337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Vesterberg O., Hansén L., Sjösten A. Staining of proteins after isoelectric focusing in gels by new procedures. Biochim Biophys Acta. 1977 Mar 28;491(1):160–166. doi: 10.1016/0005-2795(77)90052-6. [DOI] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]