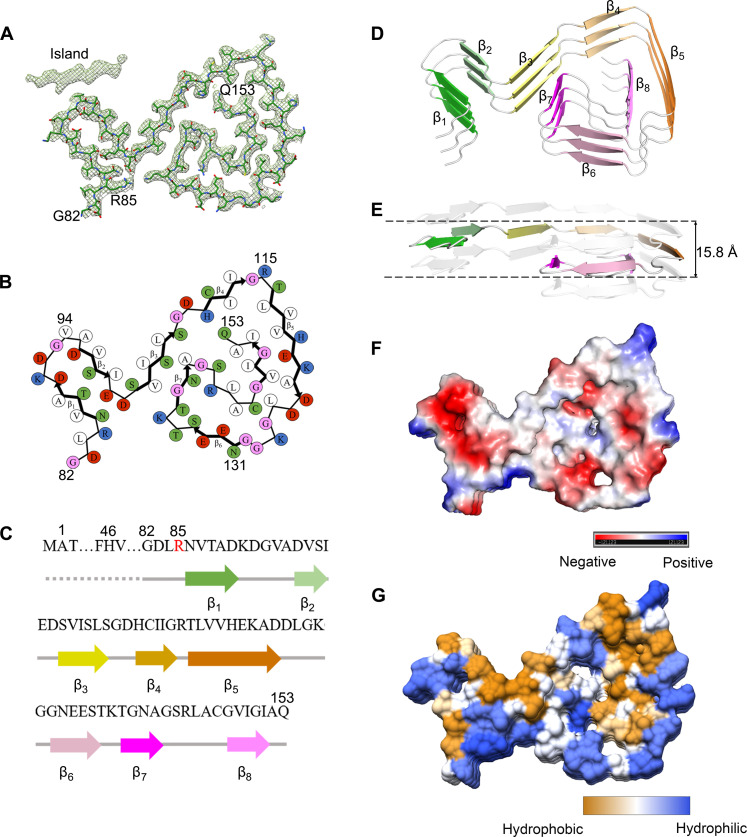
Fig. 3. The ALS-causing SOD1 mutant G85R also forms an amyloid fibril structure.
(A) Cryo-EM map of the G85R fibril with the atomic model overlaid. The G85R fibril core comprises a C-terminal segment (residues 82 to 153) colored purple. (B) Schematic view of the G85R fibril core. The residues are colored as follows: white, hydrophobic; green, polar; red and blue, negatively charged and positively charged, respectively; and magenta, glycine. β Strands are indicated with bold lines. (C) Sequence of the G85R fibril core comprising residues 82 to 153 from the full-length human G85R SOD1 (1 to 153) with the observed eight β strands colored green (β1), light green (β2), yellow (β3), gold (β4), orange (β5), pink (β6), magenta (β7), and light magenta (β8) in the C-terminal segment. The dotted line corresponds to residues 1 to 81 not modeled in the cryo-EM density. The ALS-causing mutation site R85 is highlighted in red. (D) Ribbon representation of the structure of a G85R fibril core containing three molecular layers and a C-terminal segment. We show the secondary structure panel in the same orientation as the other panels. (E) As in (D), but viewed perpendicular to the helical axis, revealing that the height of one layer along the helical axis is 15.8 Å. (F) Electrostatic surface representation of the structure of a G85R fibril core containing three molecular layers and a C-terminal segment. (G) Hydrophobic surface representation of the structure of a G85R fibril core as in (D). The surface of the G85R fibril core is shown according to the electrostatic properties (red, negatively charged; blue, positively charged) (F) or the hydrophobicity (yellow, hydrophobic; blue, hydrophilic) (G) of the residues.