Abstract
INTRODUCTION:
Opioids used to manage severe pain in acute pancreatitis (AP) might exacerbate the disease through effects on gastrointestinal and immune functions. Methylnaltrexone, a peripherally acting µ-opioid receptor antagonist, may counteract these effects without changing analgesia.
METHODS:
This double-blind, randomized, placebo-controlled trial included adult patients with AP and systemic inflammatory response syndrome at 4 Danish centers. Patients were randomized to receive 5 days of continuous intravenous methylnaltrexone (0.15 mg/kg/d) or placebo added to the standard of care. The primary end point was the Pancreatitis Activity Scoring System score after 48 hours of treatment. Main secondary outcomes included pain scores, opioid use, disease severity, and mortality.
RESULTS:
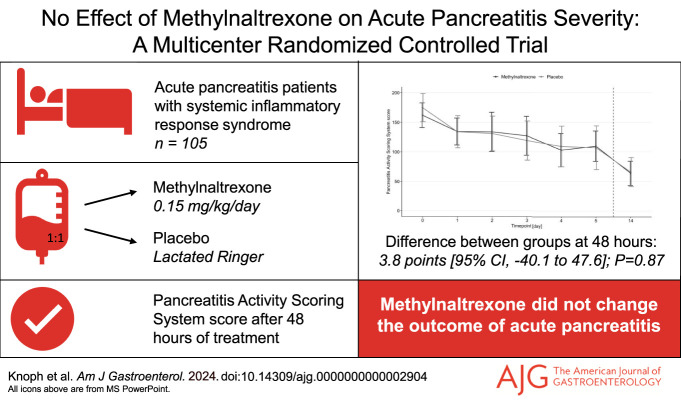
In total, 105 patients (54% men) were randomized to methylnaltrexone (n = 51) or placebo (n = 54). After 48 hours, the Pancreatitis Activity Scoring System score was 134.3 points in the methylnaltrexone group and 130.5 points in the placebo group (difference 3.8, 95% confidence interval [CI] −40.1 to 47.6; P = 0.87). At 48 hours, we found no differences between the groups in pain severity (0.0, 95% CI −0.8 to 0.9; P = 0.94), pain interference (−0.3, 95% CI −1.4 to 0.8; P = 0.55), and morphine equivalent doses (6.5 mg, 95% CI −2.1 to 15.2; P = 0.14). Methylnaltrexone also did not affect the risk of severe disease (8%, 95% CI −11 to 28; P = 0.38) and mortality (6%, 95% CI −1 to 12; P = 0.11). The medication was well tolerated.
DISCUSSION:
Methylnaltrexone treatment did not achieve superiority over placebo for reducing the severity of AP.
KEYWORDS: pancreatitis, methylnaltrexone, severity, opioids
INTRODUCTION
Acute pancreatitis (AP) has an increasing global incidence, resulting in substantial morbidity and, in severe cases, even mortality (1,2). Nevertheless, no targeted pharmacotherapy has been identified for this disease (3). Patients with AP suffer from severe epigastric pain, often warranting opioid treatment (1,4,5). Furthermore, there is preclinical evidence suggesting increased levels of endogenous opioids in patients with AP (6).
Opioids (endogenous or exogenous) exert their peripheral effects by binding to the µ-receptors in the enteric nervous system (7). Human studies found decreased gastrointestinal secretion and dysmotility during opioid treatment (8,9). The latter may result in intestinal bacterial overgrowth, which, together with an impaired intestinal permeability (10,11), may contribute to the frequent presence of enteric-derived bacteria in infected pancreatic necrosis and Gram-negative sepsis in patients with AP (12). Finally, opioids can cause spasms of the sphincter of Oddi and reduce fluid secretion (8), which may reduce the flow within the pancreatic duct system, worsen pancreatic autodigestion, and prevent the resolution of intrapancreatic inflammation.
Methylnaltrexone, a peripherally acting µ-opioid receptor antagonist with limited capacity to cross the blood-brain barrier, is indicated for opioid-induced constipation and has the potential to counteract the peripheral effects of opioids without affecting analgesia (13,14). We hypothesized that treatment with methylnaltrexone would restrict putative adverse effects of endogenous and exogenous opioids and reduce disease severity in patients with predicted moderately severe or severe AP. We aimed to examine the clinical efficacy, tolerability, and safety of methylnaltrexone compared with placebo through assessments of (i) disease severity as measured by the Pancreatitis Activity Scoring System (PASS) score, (ii) symptoms as measured by validated questionnaires, and (iii) clinical outcomes such as length of admission, the severity of AP according to the revised Atlanta Classification (5), and mortality.
METHODS
Study design and participants
This multicenter, double-blind, randomized, placebo-controlled, investigator-initiated, superiority trial was conducted at 4 Danish pancreas referral centers (Aalborg University Hospital, Aalborg; Odense University Hospital, Svendborg; and Copenhagen University Hospitals, Hvidovre and Bispebjerg). The previously published trial protocol (15) adhered to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) 2013 statement (16). The protocol is available online at ClinicalTrials.gov (Identifier: NCT04743570), where the study was registered before the inclusion of subjects. The study was approved by the North Denmark Region Committee on Health Research Ethics (Identifier: N-20200060) and the Danish Medicines Agency (EudraCT identifier: 2020-002313-18) and followed the principles of the Helsinki Declaration. All 4 recruiting centers were regularly inspected by an independent monitor appointed by the Good Clinical Practice unit at the respective sites. We screened all adult patients between 18 and 85 years admitted with AP according to the Atlanta criteria (2 of the following: severe, epigastric pain; serum amylase or lipase 3 times the upper normal limit; and characteristic AP imaging findings) (5). Patients fulfilling 2 or more systemic inflammatory response syndrome (SIRS) criteria within the past 24 hours were eligible for inclusion. The SIRS criteria were assessed based on body temperature (<36 °C or >38 °C), white blood cell count (<4,000 cells/mm3 or >12,000 cells/mm3), respiration (frequency >20/min or PaCO2 <32 mm Hg), and pulse (>90 beats per minute). Until December 2021, we excluded patients with symptoms for more than 48 hours, but this criterion was omitted due to the lack of scientific rationale for the 48-hour cutoff and stagnant enrollment. We excluded patients with an allergy to methylnaltrexone, major obstruction or perforation of the intestines, abdominal cancer, need for dialysis, severe liver cirrhosis (Child-Pugh class B or C), and pregnancy or current lactation (14). Furthermore, we excluded patients with definite chronic pancreatitis (17), preexisting renal insufficiency (habitual estimated glomerular filtration rate below 45 mL/min/1.73 m2), severe preexisting comorbidities, and severe nonpancreaticobiliary infections (15). All patients gave written informed consent.
Intervention
Patients were randomized (1:1) to receive a daily amount of either 0.15 mg/kg methylnaltrexone or a corresponding volume of placebo (lactated Ringer). This dosage aligned with the approved subcutaneous use of methylnaltrexone for opioid-induced constipation (14) and previous intravenous use for research purposes (18). The daily dose of methylnaltrexone or placebo was mixed in 1,000 mL of lactated Ringer and delivered by continuous intravenous infusion over 24 hours, which was repeated daily for a maximum of 5 days. Daily doses were reduced by 50% for severe renal impairment (estimated glomerular filtration rate <30 mL/min) during study participation, but otherwise, the daily doses were fixed based on admission weight. The infusion of methylnaltrexone or placebo was discontinued if the patient required dialysis, was discharged, put on medical leave, or transferred to another hospital. Medication compliance was monitored using the administrated volume of study medication and the weight of used vials. Randomization was conducted using random block sizes without stratification by The Hospital Pharmacy at Herlev Hospital. They were responsible for all procedures related to randomization, including generating the random allocation sequence and distributing the study medications. Study medication was packaged and labeled into vials with methylnaltrexone and placebo, having a similar appearance. Patients, study personnel, and treatment-responsible medical personnel were blinded to the allocation. During study participation, patients received unrestricted treatment according to the standard of care, following international guidelines (19). This included fluid resuscitation, analgesics (opioid and nonopioid), laxative treatment, nutrition, antibiotics, and surgical interventions, such as endoscopic retrograde cholangiopancreatography and prophylactic cholecystectomy, as prescribed by the treatment-responsible physicians.
Outcomes
The primary outcome was the PASS score after 48 hours of treatment. We choose 48 hours as the primary assessment time point since methylnaltrexone rapidly reaches a steady state with both subcutaneous and intravenous use (20,21), and at this minimum time frame, AP severity can be determined (5). The PASS score was selected for its robustness, extensive validation in AP, and simple calculation using clinical variables available from routine management (22,23). The PASS score was recorded daily based on the following parameters: the presence of organ failure (100 points per system), the number of SIRS criteria fulfilled (25 points per criterion), the maximum intensity of abdominal pain (5 points per numeric rating scale point ranging from 0 to 10), tolerance to solid diet (40 points if solid diet was not tolerated, 0 points if solid diet was tolerated), and morphine equivalent doses (5 points per intravenous morphine equivalent dose) (24). Since we studied the temporal evolution of the PASS score, we found it appropriate to select patients based on SIRS at baseline despite the SIRS being included in the PASS score. We used the Modified Marshall Scoring System to assess organ failure. For the evaluation of respiratory failure, we used peripheral capillary oxygen saturation measures when arterial blood oxygen levels were unavailable (25,26). All types of opioids administered were registered and subsequently converted to morphine-equivalent doses (27). Prespecified secondary outcomes were the AP severity (revised Atlanta Classification) (5); quantification of need for analgesics, intravenous fluids, or antibiotics; length of admission; and mortality at 30 and 90 days. Patients were asked to complete 3 validated questionnaires daily during treatment: The modified Brief Pain Inventory short form (28), the Bristol Stool Form Scale (29), and the Gastrointestinal Symptom Rating Scale (30). As the interference score of the Brief Pain Inventory questionnaire is irrelevant in an acute setting, we chose not to report this in the primary analysis. We also registered biochemistry (C-reactive protein, white blood cell count, creatinine, amylase, albumin) and the need for laxatives daily during treatment. A modified PASS score excluding the morphine equivalent doses module was included post hoc as a secondary outcome due to variability in opioid use (31). At the follow-up visit on day 14, we registered the PASS score, questionnaires, biochemistry, and quantification of analgesics, intravenous fluids, laxatives, and antibiotics. For safety evaluation, adverse events and serious adverse events were registered.
Statistical analysis
The trial was powered to detect a 25-point difference in PASS scores between groups after 48 hours of treatment, which was previously indicated to separate mild and severe AP (23,32). Based on previous research on the PASS score, we assumed a within-group SD of 40 points (23,32). With 80% power, and a 2-sided α level of 0.05, the power calculation required 41 patients per group, and the prespecified sample size was 90 patients. Descriptive statistics for continuous variables were expressed as mean ± SD or median (interquartile range [IQR]) depending on normality, which was assessed by the Shapiro-Wilk test. Categorical data were presented as numbers and proportions. The primary analysis was by intention-to-treat and involved data collected within the treatment period of all patients who underwent randomization (numbers at risk, Figure 2). Variables with missing data underwent multiple imputation before analysis. For the primary outcome, differences between treatment groups were tested using a robust linear mixed model of repeated measures. We included terms for the assessment time point, treatment group, and interaction between the assessment time points and treatment group. From this model, we extracted point estimates for the mean PASS score, SEM, and the mean difference with a 95% confidence interval (CI) after 48 hours (primary end point). Secondary outcomes that were measured repeatedly were analyzed using a robust linear mixed model as for the primary outcome. Single time point measurements of continuous data were analyzed by the Student t test or Wilcoxon rank-sum test, and the results were presented as the mean difference with 95% CI. Categorical data were analyzed using the χ2 test or Fisher exact test as appropriate, and the results were presented as the risk differences with a 95% CI. In addition to the intention-to-treat analysis, we performed an analysis of the per-protocol population (prespecified as ≥75% treatment compliance within the first 48 hours of treatment) and a complete case analysis of the intention-to-treat population. We also performed an intention-to-treat analysis of change-from-baseline data. Multiple imputation was performed using the package “mice” in R studio with predictive mean matching for continuous data and logistic regression for binary outcomes. Statistical significance was defined as a 2-sided P value below 0.05. All statistical methods were prespecified in the statistical analysis plan (available online at ClinicalTrials.gov) except for the handling of missing data, including the multiple imputation approach and complete case analysis. Statistical analyses were performed using STATA (version 16.0) and R studio (version 4.3.0, packages: tidyverse, ggplot2, ggpubr, mice, lubridate, and haven).
Figure 2.
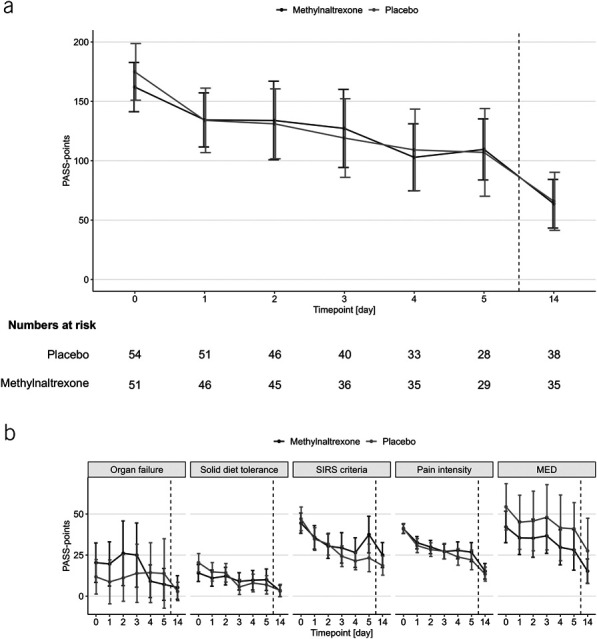
PASS scores in the intention-to-treat population. Line graphs show means and 95% CIs (whiskers) of daily PASS scores (a) and PASS points within the 5 scoring elements (b). Data are shown for the intention-to-treat population. Missing data were imputed using the multiple imputation approach. The dashed lines separate the end of treatment and follow-up on day 14. CI, confidence interval; MED, morphine equivalent doses; PASS, Pancreatitis Activity Scoring System; SIRS, systemic inflammatory response syndrome.
RESULTS
Participant flow
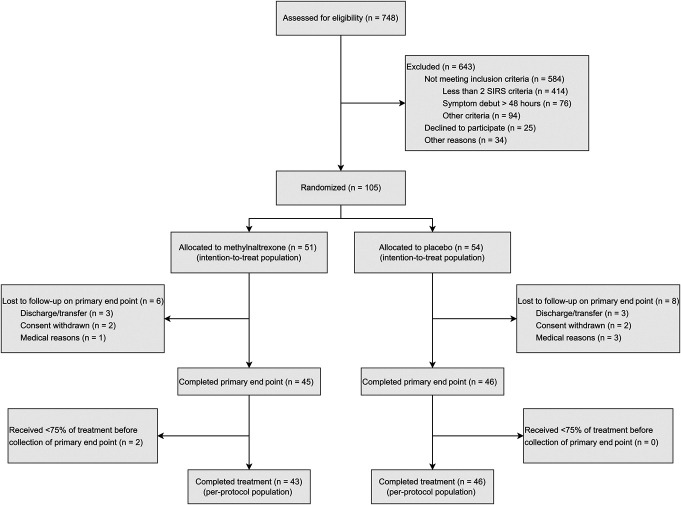
Of 748 patients with AP assessed for eligibility, 105 were randomized between May 14, 2021, and April 9, 2023, as illustrated in Figure 1. In total, 51 patients were allocated to methylnaltrexone, and 54 patients were allocated to placebo (intention-to-treat population). The trial concluded with primary end point data from 91 patients, of which 45 patients were in the methylnaltrexone group and 46 patients were in the placebo group. The per-protocol population had 43 patients in the methylnaltrexone group and 46 patients in the placebo group.
Figure 1.
Flowchart for screening, recruitment, randomization, and follow-up in the trial. SIRS, systemic inflammatory response syndrome.
Baseline characteristics
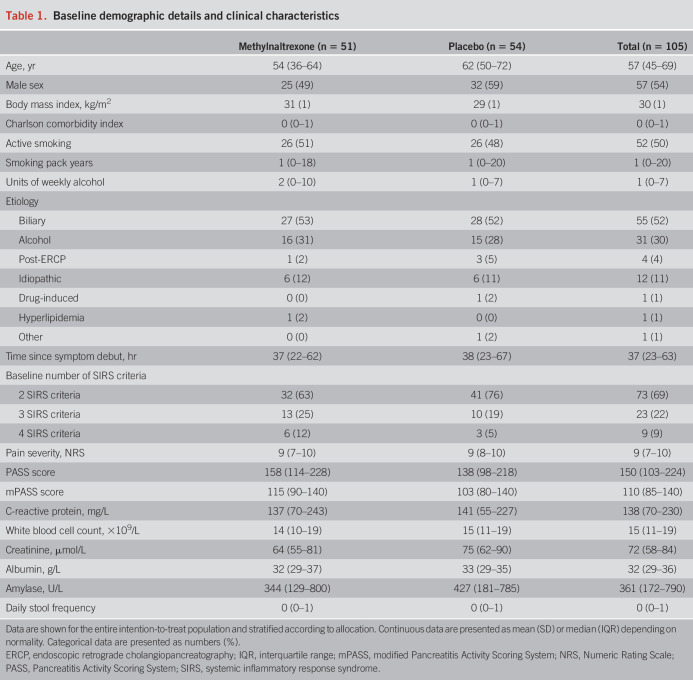
The median age in the intention-to-treat population was 57 (IQR 45–69) years, and 57 patients (54%) were male. Biliary etiology, which occurred in 55 patients (52%), was the most common, followed by alcoholic AP, which was found in 31 patients (30%). The median baseline PASS score was 158 (IQR 114–228) points in the methylnaltrexone group and 138 (IQR 98–218) points in the placebo group. The median time from symptom debut to inclusion was 37 (IQR 22–62) hours in the methylnaltrexone group and 38 (IQR 23–67) hours in the placebo group. Baseline demographic details and clinical characteristics were balanced between groups (Table 1).
Table 1.
Baseline demographic details and clinical characteristics
Primary outcome
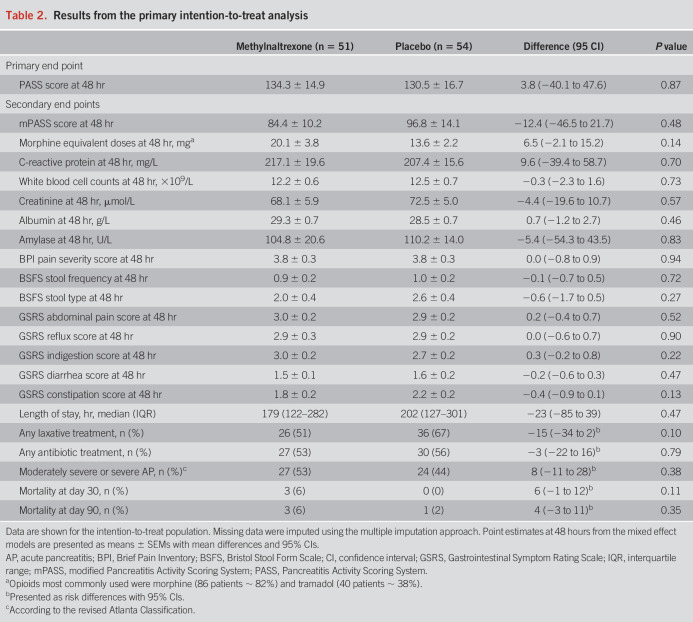
At 48 hours, the estimated mean PASS score was 134.3 points in the methylnaltrexone group and 130.5 points in the placebo group (difference 3.8 points, 95% CI −40.1 to 47.6; P = 0.87) (Table 2 and Figure 2a). This similarity between groups seemed balanced across all 5 scoring elements, including organ failure scores (Figure 2b).
Table 2.
Results from the primary intention-to-treat analysis
Secondary outcomes
Secondary outcomes are reported in Table 2. The estimated mean modified PASS score at 48 hours of treatment was 84.4 points in the methylnaltrexone group compared with 96.8 points in the placebo group (difference −12.4 points, 95% CI −46.5 to 21.7). Estimated mean morphine equivalent doses at 48 hours were 20.1 mg in the methylnaltrexone group and 13.6 mg in the placebo group (difference 6.5 mg, 95% CI −2.1 to 15.2). At 48 hours, groups reported similar pain severity (difference 0.0, 95% CI −0.8 to 0.9) and pain interference (difference −0.3, 95% CI −1.4 to 0.8) scores from the modified Brief Pain Inventory short form. In the methylnaltrexone group, 26 patients (51%) received laxative treatment during study participation, while 36 patients (67%) required this in the placebo group (risk difference −15%, 95% CI −34 to 2). The median length of admission was 179 hours in the methylnaltrexone group compared with 202 hours in the placebo group (difference −23 hours, 95% CI −85 to 39). In the methylnaltrexone group, 27 patients (53%) developed moderately severe or severe AP, while this was 24 patients (44%) in the placebo group (risk difference 8%, 95% CI −11 to 28). Mortality was similar between groups at day 30 (risk difference 6%, 95% CI −1 to 12) and day 90 (risk difference 4%, 95% CI −3 to 11). Biochemical parameters, antibiotic use, and gastrointestinal symptoms (assessed using the Bristol Stool Form Scale and the Gastrointestinal Symptom Rating Scale) showed no difference between groups (Table 2 and in the Supplementary Figures S1–S4, see Supplementary Digital Content 1, http://links.lww.com/AJG/D316). In the methylnaltrexone group, 5 patients (10%) received no opioid during study participation, whereas this was the case for 4 patients (7%) in the placebo group. The per-protocol and complete-case analyses, along with the intention-to-treat analysis using change-from-baseline data, are presented in Supplementary Tables S1–S3 and Figures S1–S5 (see Supplementary Digital Content 1, http://links.lww.com/AJG/D316). The results of these analyses were comparable with the primary results of the intention-to-treat population using the multiple imputation approach.
Safety and tolerability
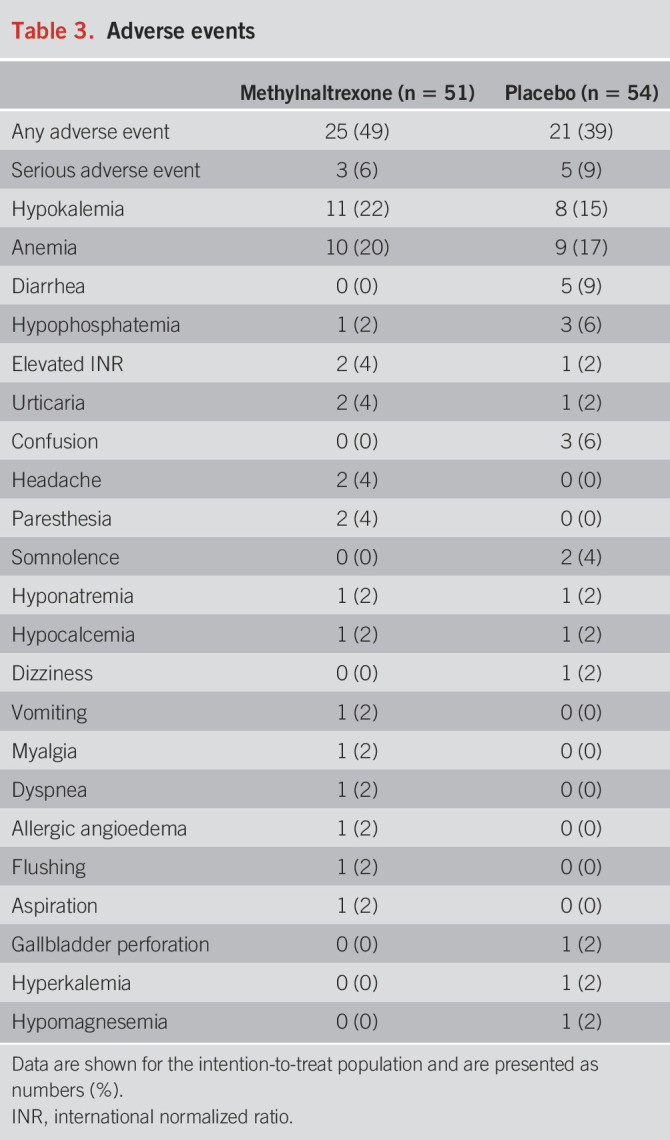
Adverse events were observed in 25 patients (49%) in the methylnaltrexone group and 21 patients (39%) in the placebo group (Table 3). Serious adverse events occurred in 3 patients (6%) with methylnaltrexone and included laparoscopic cholecystectomy, aspiration, and suspected allergy. In the placebo group, serious adverse events occurred in 5 patients (9%) and included sepsis, elevated international normalized ratio, laparoscopic cholecystectomy, pneumonia, and gallbladder perforation. None were deemed related to methylnaltrexone treatment. The most common adverse events were hypokalemia, anemia, and diarrhea.
Table 3.
Adverse events
DISCUSSION
In this multicenter, randomized, placebo-controlled trial of 105 AP patients with SIRS, continuous intravenous methylnaltrexone did not achieve superiority over placebo for reducing disease severity. Furthermore, the medication did not seem to affect symptoms or outcomes of the disease but was well tolerated.
To the best of our knowledge, methylnaltrexone has not previously been tested in a cohort of patients with AP, and the proper dose and administration regimens have not been established in this setting. Daily or bidaily subcutaneous boluses of 0.15 mg/kg methylnaltrexone have consistently been shown to relieve opioid-induced constipation, aligning with the approved use for methylnaltrexone (13,14,33,34). In previous studies, intravenous boluses of 0.3 mg/kg methylnaltrexone accelerated postoperative bowel recovery (35) and reduced transit times in healthy subjects (20). However, intravenous methylnaltrexone boluses of 0.15 mg/kg did not promote laxation in critical care patients on stable opioid treatment (18). The dose chosen for this study may have been inadequate for effective opioid antagonism in the gut. However, this is unlikely as methylnaltrexone has a significantly higher affinity for the µ-opioid receptor compared with conventional opioids, blocking up to 97% of morphine's effect on intestinal motility (36,37). The continuous mode of delivery chosen for this study might have led to lower peak concentrations, potentially also affecting the effect of the drug. We chose to administer methylnaltrexone continuously to prevent adverse reactions, including abdominal cramps and nausea, which are commonly reported with the bolus regimens (13,33). Throughout the study, we observed numerically higher morphine equivalent doses in the methylnaltrexone group compared with placebo. Since it is well established that methylnaltrexone has a restricted ability to cross the blood-brain barrier, it is unlikely that this is due to central opioid antagonism (21).
In this study, we found no difference in stool frequencies between patients receiving methylnaltrexone and placebo, contradicting the anticipated mode of action for methylnaltrexone. The premise of this study was that opioids may cause dysmotility, which could be one of the mechanisms to increase severity in AP. However, numerous potential modifiers of gastrointestinal motility are present in AP and were not controlled for in this study (38), potentially impacting bowel movements. In AP with critical illness, paralytic ileus may be caused by metabolic derangement, fluid imbalances, and severe infections (39). Preclinical studies have indicated that systemic inflammation, which is a hallmark of AP, can cause ileus in itself through chemokine secretion to the intestinal lumen (40,41). Furthermore, dysmotility may result from neuron damage or gut hormone secretion in AP (42,43). Since concurrent medical treatment was unrestricted in this study, factors such as laxative use may also have impacted gastrointestinal motility. Although dysmotility has been linked to severe AP, clinical evidence has also indicated that dysmotility is present even in mild cases (43,44). This may be why we observe such a high need for laxatives in our study population, even in the methylnaltrexone group.
As discussed above, AP is a complex setting of coexisting conditions in which the role of endogenous and exogenous opioids remains incompletely understood. The safety of opioids in AP has been continuously debated since opioids can interfere with motility, intestinal and pancreatic secretion, tone in the sphincters, and inflammation in a negative manner. Retrospective clinical studies previously found that opioids increased the risk of 30-day mortality, organ-supportive treatment, longer admission length, and aggravated morphologic AP severity (4,45,46). By contrast, other studies indicated no difference in the risk of severe disease between opioids and other analgesics in AP (47,48). Investigating the safety of opioids in AP through a randomized comparison with a placebo is ethically challenging due to potential harm to patients in the placebo arm and the predominant use of opioids as rescue analgesia in AP trials (49). We have previously shown that the peripheral effects of opioids can be antagonized by peripherally restricted opioid antagonists (50–53). Although indirectly, this study did not support that opioids have peripheral harmful effects in AP.
A strength of our study was the prospective, randomized, and placebo-controlled trial design. Furthermore, the composition of our cohort aligned with previous reports on AP patients with regard to age, etiology, morbidity, and mortality (1,54). There are several limitations. At baseline, we observed a 20-point higher PASS score in the methylnaltrexone group, indicating that our groups were not sufficiently balanced at randomization. However, the change-from-baseline sensitivity analysis gave the same results as the primary analysis. During the study, we removed the exclusion criteria of symptoms for more than 48 hours, as most screened patients were admitted to the hospital with longer-lasting pain. Ideally, treatment should be initiated directly on symptom onset, but this was not realistic as pain often develops over time, and many patients likely recover spontaneously. Approximately half of the patients in this study had mild AP despite being selected using the SIRS criteria, which aligns with prior findings on AP severity prediction accuracy (55). However, we do recognize that this high proportion of mild AP may have weakened the impact of methylnaltrexone on disease severity, potentially underpowering the study.
In summary, continuous intravenous methylnaltrexone infusions did not demonstrate superiority over placebo for reducing disease severity in AP patients with SIRS.
CONFLICTS OF INTEREST
Guarantor of the article: Asbjørn Mohr Drewes, MD, PhD, DMSc.
Specific author contributions: C.S.K., S.S.O., and A.M.D. had direct access to and verified the underlying data reported in this manuscript. C.S.K., M.E.C., S.N., M.B.H., M.B.M., L.B.J.N., O.T.U., J.B.F., S.S.O., and A.M.D.: conceptualization and methodology. C.S.K., M.E.C., S.N., M.B.H., M.B.M., L.B.J.N., I.M.H., C.S., C.E.L.N., A.J.A., S.P., L.S.S., O.T.U., J.B.F., S.S.O., and A.M.D.: investigation, resources, and validation. C.S.K., S.S.O., and A.M.D. supported by S.N., M.B.H., M.B.M., S.P., O.T.U., and J.B.F.: software, formal analysis, visualization, and data curation. C.S.K., S.S.O., and A.M.D.: supervision, project administration, and funding acquisition. C.S.K., S.S.O., and A.M.D.: writing—original draft. C.S.K., M.E.C., S.N., M.B.H., M.B.M., L.B.J.N., I.M.H., C.S., C.E.L.N., A.J.A., S.P., L.S.S., O.T.U., J.B.F., S.S.O., and A.M.D.: writing—review & editing including final approval.
Financial support: The study was funded by The Novo Nordisk Foundation, grant#NNF19OC0057331. The Novo Nordisk Foundation had no role in the study design, data collection, data analysis, data interpretation, or writing the report.
Potential competing interests: None to report.
Clinical trial registration: ClinicalTrials.gov, trial identification number: NCT04743570.
Ethics: The study was approved by the North Denmark Region Committee on Health Research Ethics (Identifier: N-20200060) and the Danish Medicines Agency (EudraCT identifier: 2020-002313-18) and followed the principles of the Helsinki Declaration. All participants gave written informed consent.
Data availability statement: Data that support the findings of this trial are available from the corresponding author upon reasonable request.
Study Highlights.
WHAT IS KNOWN
✓ Opioid sparing is often recommended for acute pancreatitis (AP) pain due to safety concerns.
✓ Methylnaltrexone, a peripheral µ-opioid receptor antagonist, may counteract peripheral effects of opioid administration without affecting analgesia.
✓ Methylnaltrexone has never been investigated in AP.
WHAT IS NEW HERE
✓ Forty-eight hours of continuous intravenous methylnaltrexone treatment did not achieve superiority over placebo for reducing the severity of AP, as measured by the Pancreatitis Activity Scoring System score.
✓ Methylnaltrexone treatment was well tolerated by the patients in this study.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the nursing staff at our 4 study sites and the patients who opted to participate in the study. We also wish to recognize our collaborators for their invaluable contributions. Annette Aggerholm Overbye (Department of Gastrointestinal Surgery, Aalborg University Hospital, Denmark), Ann Hauberg (Department of Gastrointestinal Surgery, Aalborg University Hospital, Denmark), Mohamad Ali Abdul Ghani (Department of Surgery, Odense University Hospital, Svendborg, Denmark), Sandie Jönch Møller (Digestive Disease Center K, Bispebjerg University Hospital of Copenhagen, Denmark), Mette Brogaard Barrit (Digestive Disease Center K, Bispebjerg University Hospital of Copenhagen, Denmark), Karen Lisa Hilsted (Gastrounit, Hvidovre University Hospital of Copenhagen, Denmark), Camilla Møller Vorsholt (Gastrounit, Hvidovre University Hospital of Copenhagen, Denmark), Joy Stinne Timmner (Gastrounit, Hvidovre University Hospital of Copenhagen, Denmark), Morten Laksáfoss Lauritsen (Gastrounit, Hvidovre University Hospital of Copenhagen, Denmark).
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/D316
Contributor Information
Cecilie Siggaard Knoph, Email: c.siggaard@rn.dk.
Mathias Ellgaard Cook, Email: m.cook@rn.dk.
Srdan Novovic, Email: srdan.novovic@regionh.dk.
Mark Berner Hansen, Email: MBHansen@zealandpharma.com.
Michael Bau Mortensen, Email: michael.bau.mortensen@rsyd.dk.
Liv Bjerre Juul Nielsen, Email: liv.bjerre.juul.nielsen@regionh.dk.
Irene Maria Høgsberg, Email: irene.maria.hoegsberg@rsyd.dk.
Celina Salomon, Email: Celina.salomon@rsyd.dk.
Celine Emilie Lindqvist Neergaard, Email: Celine.Neergaard@rsyd.dk.
Aseel Jabbar Aajwad, Email: a.jabbar@rn.dk.
Sanjay Pandanaboyana, Email: s.pandanaboyana@nhs.net.
Lone Schmidt Sørensen, Email: lss@rn.dk.
Ole Thorlacius-Ussing, Email: otu@rn.dk.
Jens Brøndum Frøkjær, Email: jebf@rn.dk.
Søren Schou Olesen, Email: soso@rn.dk.
REFERENCES
- 1.Szatmary P, Grammatikopoulos T, Cai W, et al. Acute pancreatitis: Diagnosis and treatment. Drugs 2022;82(12):1251–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iannuzzi JP, King JA, Leong JH, et al. Global incidence of acute pancreatitis is increasing over time: A systematic review and meta-analysis. Gastroenterology 2022;162(1):122–34. [DOI] [PubMed] [Google Scholar]
- 3.Moggia E, Koti R, Belgaumkar AP, et al. Pharmacological interventions for acute pancreatitis. Cochrane Database Syst Rev 2017;4(4):CD011384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu BU, Butler RK, Chen W. Factors associated with opioid use in patients hospitalized for acute pancreatitis. JAMA Netw Open 2019;2(4):e191827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013;62(1):102–11. [DOI] [PubMed] [Google Scholar]
- 6.Satake K, Ha SS, Hiura A, et al. The therapeutic effect of a new synthetic protease inhibitor (E-3123) on hemodynamic changes during experimental acute pancreatitis in dogs. Gastroenterol Jpn 1993;28(1):64–71. [DOI] [PubMed] [Google Scholar]
- 7.Sternini C, Patierno S, Selmer IS, et al. The opioid system in the gastrointestinal tract. Neurogastroenterol Motil 2004;16(Suppl 2):3–16. [DOI] [PubMed] [Google Scholar]
- 8.Drewes AM, Munkholm P, Simrén M, et al. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction-Recommendations of the Nordic Working Group. Scand J Pain 2016;11:111–22. [DOI] [PubMed] [Google Scholar]
- 9.Mark EB, Bødker MB, Grønlund D, et al. MRI analysis of fecal volume and dryness: Validation study using an experimental oxycodone-induced constipation model. J Magn Reson Imaging 2019;50(3):733–45. [DOI] [PubMed] [Google Scholar]
- 10.Barlass U, Dutta R, Cheema H, et al. Morphine worsens the severity and prevents pancreatic regeneration in mouse models of acute pancreatitis. Gut 2018;67(4):600–2. [DOI] [PubMed] [Google Scholar]
- 11.Meng J, Yu H, Ma J, et al. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One 2013;8(1):e54040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas 2003;26(2):122–9. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain BH, Rhiner M, Slatkin NE, et al. Subcutaneous methylnaltrexone for opioid-induced constipation in advanced-illness patients with or without active cancer. Pain Manag 2020;10(2):73–84. [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency. Annex I: Summary of product characteristics (Relistor) (https://www.ema.europa.eu/en/documents/product-information/relistor-epar-product-information_en.pdf) (2008).
- 15.Knoph CS, Cook ME, Fjelsted CA, et al. Effects of the peripherally acting μ-opioid receptor antagonist methylnaltrexone on acute pancreatitis severity: Study protocol for a multicentre double-blind randomised placebo-controlled interventional trial, the PAMORA-AP trial. Trials 2021;22(1):940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann Intern Med 2013;158(3):200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider A, Löhr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: Introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol 2007;42(2):101–19. [DOI] [PubMed] [Google Scholar]
- 18.Patel PB, Brett SJ, O'Callaghan D, et al. Methylnaltrexone for the treatment of opioid-induced constipation and gastrointestinal stasis in intensive care patients. Results from the MOTION trial. Intensive Care Med 2020;46(4):747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13(4 Suppl 2):e1–15. [DOI] [PubMed] [Google Scholar]
- 20.Yuan CS, Doshan H, Charney MR, et al. Tolerability, gut effects, and pharmacokinetics of methylnaltrexone following repeated intravenous administration in humans. J Clin Pharmacol 2005;45(5):538–46. [DOI] [PubMed] [Google Scholar]
- 21.Rotshteyn Y, Boyd TA, Yuan CS. Methylnaltrexone bromide: Research update of pharmacokinetics following parenteral administration. Expert Opin Drug Metab Toxicol 2011;7(2):227–35. [DOI] [PubMed] [Google Scholar]
- 22.Paragomi P, Tuft M, Pothoulakis I, et al. Dynamic changes in the pancreatitis activity scoring system during hospital course in a multicenter, prospective cohort. J Gastroenterol Hepatol 2021;36(9):2416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buxbaum J, Quezada M, Chong B, et al. The Pancreatitis Activity Scoring System predicts clinical outcomes in acute pancreatitis: Findings from a prospective cohort study. Am J Gastroenterol 2018;113(5):755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu BU, Batech M, Quezada M, et al. Dynamic measurement of disease activity in acute pancreatitis: The pancreatitis activity scoring system. Am J Gastroenterol 2017;112(7):1144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007;132(2):410–7. [DOI] [PubMed] [Google Scholar]
- 26.Pandharipande PP, Shintani AK, Hagerman HE, et al. Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit Care Med 2009;37(4):1317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Iaco F, Mannaioni G, Serra S, et al. Equianalgesic, opioid switch and opioid association in different clinical settings: A narrative review. Eur Rev Med Pharmacol Sci 2022;26(6):2000–17. [DOI] [PubMed] [Google Scholar]
- 28.Mendoza T, Mayne T, Rublee D, et al. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain 2006;10(4):353–61. [DOI] [PubMed] [Google Scholar]
- 29.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32(9):920–4. [DOI] [PubMed] [Google Scholar]
- 30.Dimenäs E, Carlsson G, Glise H, et al. Relevance of norm values as part of the documentation of quality of life instruments for use in upper gastrointestinal disease. Scand J Gastroenterol Suppl 1996;221:8–13. [DOI] [PubMed] [Google Scholar]
- 31.Paragomi P, Hinton A, Pothoulakis I, et al. The modified pancreatitis activity scoring system shows distinct trajectories in acute pancreatitis: An international study. Clin Gastroenterol Hepatol 2022;20(6):1334–42.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu F, Hu X, Li SL. Exploring the value of early laboratory indicators combined with pancreatitis activity scoring system in assessing the severity and prognosis of acute pancreatitis. Pakistan J Med Sci 2023;39(5):1462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med 2008;358(22):2332–43. [DOI] [PubMed] [Google Scholar]
- 34.Chamberlain BH, Cross K, Winston JL, et al. Methylnaltrexone treatment of opioid-induced constipation in patients with advanced illness. J Pain Symptom Manage 2009;38(5):683–90. [DOI] [PubMed] [Google Scholar]
- 35.Viscusi ER, Rathmell JP, Fichera A, et al. Randomized placebo-controlled study of intravenous methylnaltrexone in postoperative ileus. J Drug Assess 2013;2(1):127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan CS, Foss JF, Moss J. Effects of methylnaltrexone on morphine-induced inhibition of contraction in isolated Guinea-pig ileum and human intestine. Eur J Pharmacol 1995;276(1–2):107–11. [DOI] [PubMed] [Google Scholar]
- 37.Moss J, Rosow CE. Development of peripheral opioid antagonists' new insights into opioid effects. Mayo Clin Proc 2008;83(10):1116–30. [DOI] [PubMed] [Google Scholar]
- 38.Umbrello M, Venco R, Palandri C, et al. Peripherally-active mu-opioid receptor antagonists for constipation in critically ill patients receiving opioids: A case-series and a systematic review and meta-analysis of the literature. Neurogastroenterol Motil 2023;35(12):e14694. [DOI] [PubMed] [Google Scholar]
- 39.Salamone S, Liu R, Staller K. Gastrointestinal dysmotility in critically ill patients: Bridging the gap between evidence and common misconceptions. J Clin Gastroenterol 2023;57(5):440–50. [DOI] [PubMed] [Google Scholar]
- 40.Castro M, Valero MS, López-Tofiño Y, et al. Radiographic and histopathological study of gastrointestinal dysmotility in lipopolysaccharide-induced sepsis in the rat. Neurogastroenterol Motil 2023;35(10):e14639. [DOI] [PubMed] [Google Scholar]
- 41.Sonnier DI, Bailey SR, Schuster RM, et al. Proinflammatory chemokines in the intestinal lumen contribute to intestinal dysfunction during endotoxemia. Shock 2012;37(1):63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou H, Liu L, Bai Y, et al. Damage of the interstitial cells of Cajal and myenteric neurons causing ileus in acute necrotizing pancreatitis rats. Surgery 2011;149(2):262–75. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Gong Z, Wu K, et al. Gastrointestinal dysmotility in patients with acute pancreatitis. J Gastroenterol Hepatol 2003;18(1):57–62. [DOI] [PubMed] [Google Scholar]
- 44.Chen CY, Lu CL, Chang FY, et al. Endothelin-1 is a candidate mediating intestinal dysmotility in patients with acute pancreatitis. Dig Dis Sci 1999;44(5):922–6. [DOI] [PubMed] [Google Scholar]
- 45.Elias A, Korytny A, Klein A, et al. The association between opioid use and opioid type and the clinical course and outcomes of acute pancreatitis. Pancreas 2022;51(5):523–30. [DOI] [PubMed] [Google Scholar]
- 46.Ashok A, Faghih M, Azadi JR, et al. Morphologic severity of acute pancreatitis on imaging is independently associated with opioid dose requirements in hospitalized patients. Dig Dis Sci 2022;67(4):1362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basurto Ona X, Rigau Comas D, Urrútia G. Opioids for acute pancreatitis pain. Cochrane Database Syst Rev 2013;2013(7):CD009179. [DOI] [PubMed] [Google Scholar]
- 48.Pandanaboyana S, Knoph CS, Olesen SS, et al. Opioid analgesia and severity of acute pancreatitis: An international multicentre cohort study on pain management in acute pancreatitis. United European Gastroenterol J 2024;12(3):326–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh DR, Mehta A, Dangol UMS. Controversies in the management of acute pancreatitis. Kathmandu Univ Med J (KUMJ) 2004;2(3):203–7. [PubMed] [Google Scholar]
- 50.Janku F, Johnson LK, Karp DD, et al. Treatment with methylnaltrexone is associated with increased survival in patients with advanced cancer. Ann Oncol 2018;29(4):1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poulsen JL, Mark EB, Brock C, et al. Colorectal transit and volume during treatment with prolonged-release oxycodone/naloxone versus oxycodone plus macrogol 3350. J Neurogastroenterol Motil 2018;24(1):119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poulsen JL, Brock C, Olesen AE, et al. Clinical potential of naloxegol in the management of opioid-induced bowel dysfunction. Clin Exp Gastroenterol 2014;7:345–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poulsen JL, Brock C, Grønlund D, et al. Prolonged-release oxycodone/naloxone improves anal sphincter relaxation compared to oxycodone plus macrogol 3350. Dig Dis Sci 2017;62(11):3156–66. [DOI] [PubMed] [Google Scholar]
- 54.Spanier BWM, Dijkgraaf MGW, Bruno MJ. Epidemiology, aetiology and outcome of acute and chronic pancreatitis: An update. Best Pract Res Clin Gastroenterol 2008;22(1):45–63. [DOI] [PubMed] [Google Scholar]
- 55.Capurso G, Ponz de Leon Pisani R, Lauri G, et al. Clinical usefulness of scoring systems to predict severe acute pancreatitis: A systematic review and meta-analysis with pre and post-test probability assessment. United European Gastroenterol J 2023;11(9):825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.