Abstract
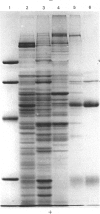
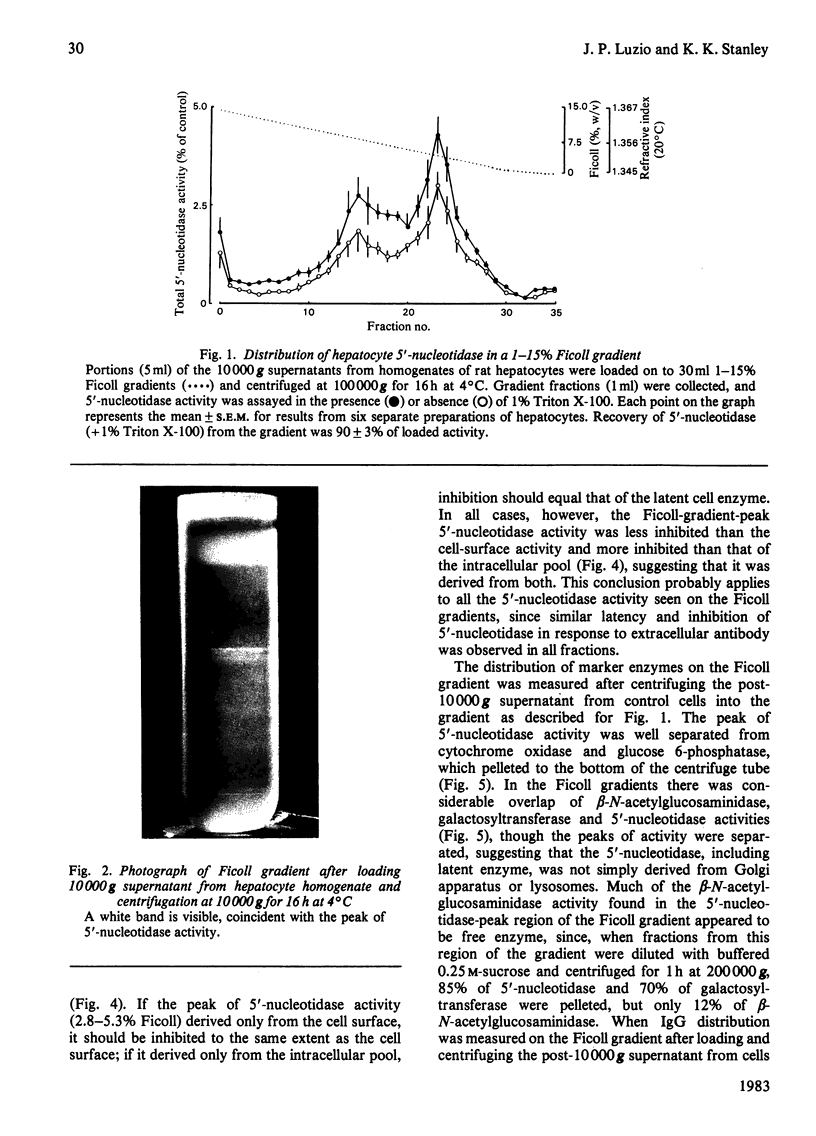
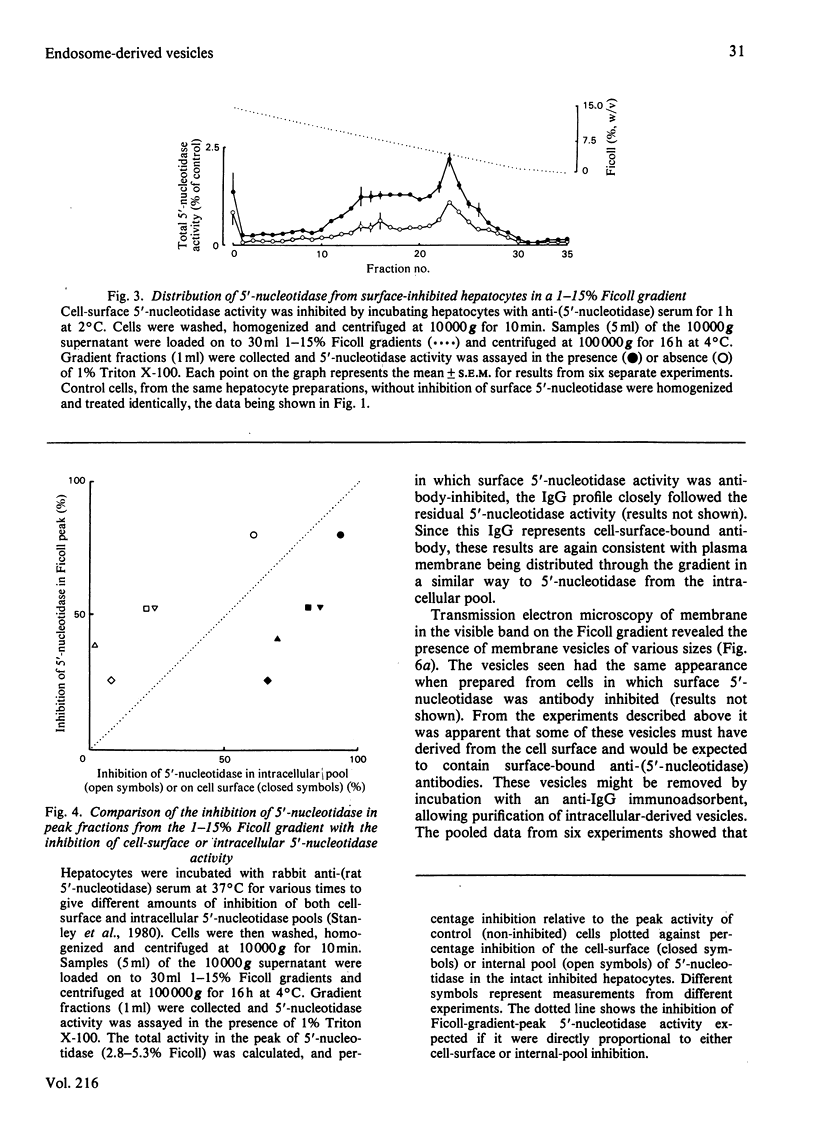
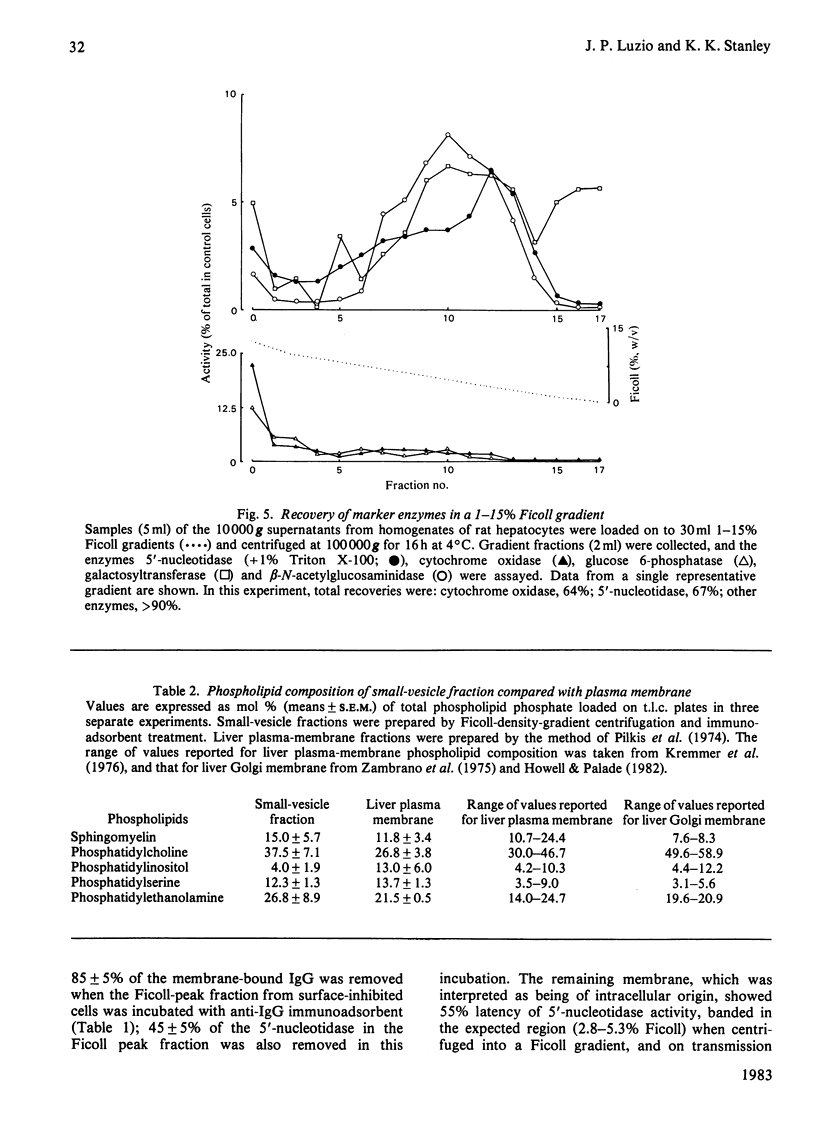
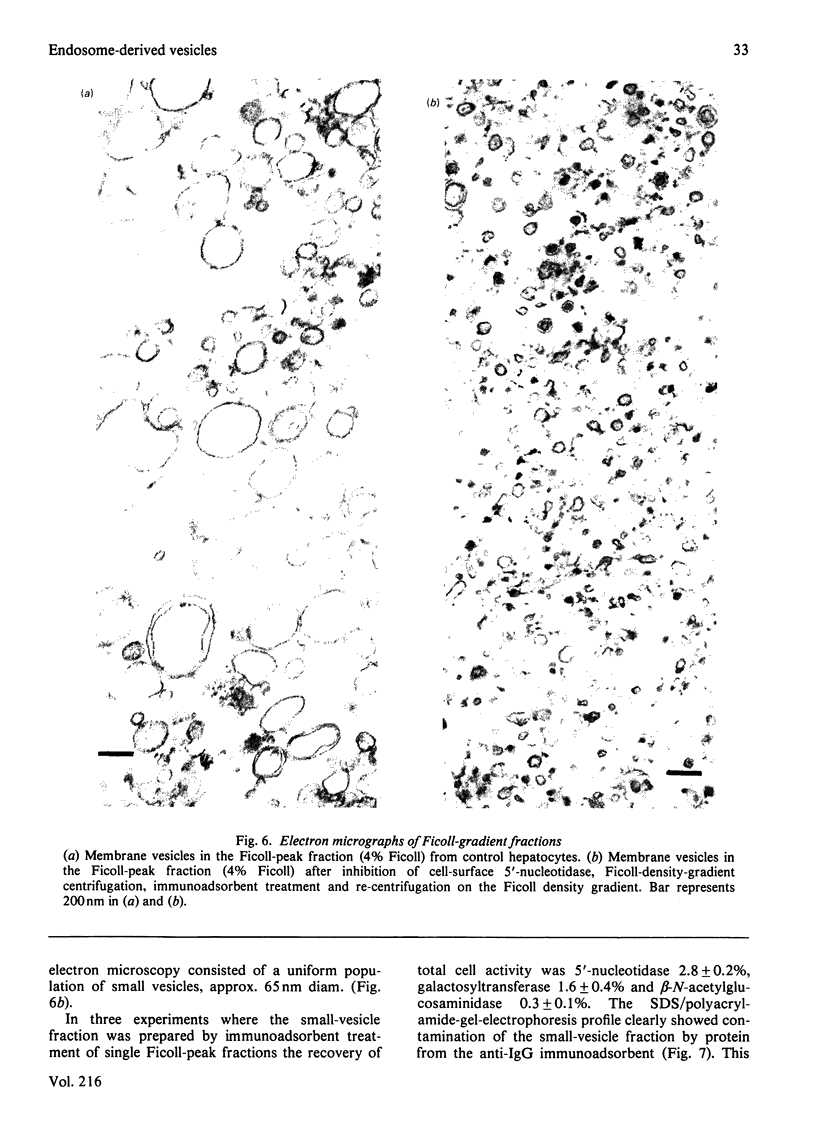
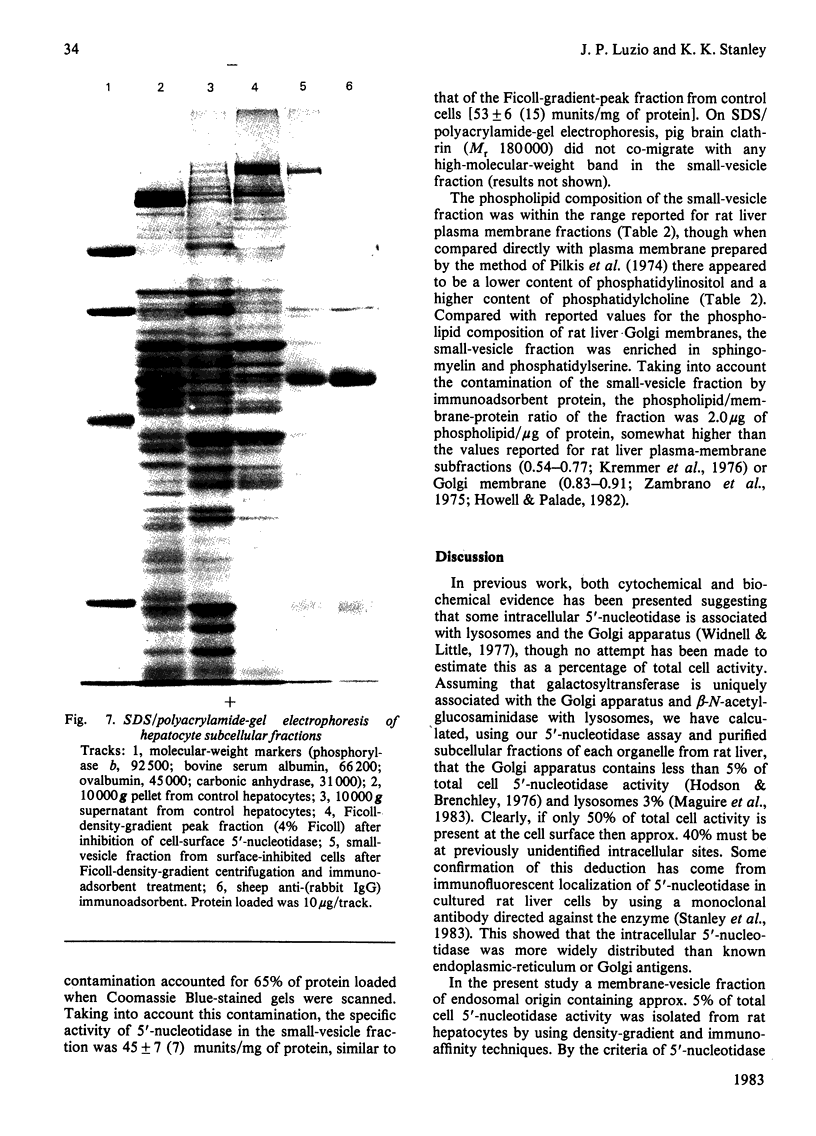
Intracellular 5'-nucleotidase involved in membrane circulation in rat hepatocytes is latent, and is protected from inhibition when whole cells are incubated with inhibiting antiserum at 2 degrees C [Stanley, Edwards & Luzio (1980) Biochem. J. 186, 59-69]. These two criteria were used to identify intracellular membrane vesicles containing 5'-nucleotidase on Ficoll density gradients. A sharply defined turbid band containing intracellular 5'-nucleotidase isolated on density gradients was further fractionated by immunoadsorption of plasma-membrane fragments derived from the cell surface of surface-inhibited cells on to an anti-(immunoglobulin G) immunoadsorbent. The resulting non-adsorbed membrane fraction consisted of vesicles of uniform size (approx. 65 nm diam.), but was not identifiable as any known organelle. This fraction could account for approx. 5% of the total cell 5'-nucleotidase activity, and the enzyme activity measured was 55% latent. The fraction had a restricted polypeptide composition but similar phospholipid composition compared with plasma membrane. We suggest that the vesicles observed in this fraction were derived from the endocytic pathway.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Bailyes E. M., Newby A. C., Siddle K., Luzio J. P. Solubilization and purification of rat liver 5'-nucleotidase by use of a zwitterionic detergent and a monoclonal-antibody immunoadsorbent. Biochem J. 1982 Apr 1;203(1):245–251. doi: 10.1042/bj2030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P., Nicholas H. Immunoassay of serum polypeptide hormones by using 125I-labelled anti(-immunoglobulin G) antibodies. Biochem J. 1975 Mar;145(3):607–616. doi: 10.1042/bj1450607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz R., Bretz H., Palade G. E. Distribution of terminal glycosyltransferases in hepatic Golgi fractions. J Cell Biol. 1980 Jan;84(1):87–101. doi: 10.1083/jcb.84.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPERSTEIN S. J., LAZAROW A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951 Apr;189(2):665–670. [PubMed] [Google Scholar]
- Debanne M. T., Evans W. H., Flint N., Regoeczi E. Receptor-rich intracellular membrane vesicles transporting asialotransferrin and insulin in liver. Nature. 1982 Jul 22;298(5872):398–400. doi: 10.1038/298398a0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fleischer B. Orientation of glycoprotein galactosyltransferase and sialyltransferase enzymes in vesicles derived from rat liver Golgi apparatus. J Cell Biol. 1981 May;89(2):246–255. doi: 10.1083/jcb.89.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Receptor-mediated endocytosis and the cellular uptake of low density lipoprotein. Ciba Found Symp. 1982;(92):77–95. doi: 10.1002/9780470720745.ch5. [DOI] [PubMed] [Google Scholar]
- Hodson S., Brenchley G. Similarities of the Golgi apparatus membrane and the plasma membrane in rat liver cells. J Cell Sci. 1976 Jan;20(1):167–182. doi: 10.1242/jcs.20.1.167. [DOI] [PubMed] [Google Scholar]
- Howell K. E., Palade G. E. Hepatic Golgi fractions resolved into membrane and content subfractions. J Cell Biol. 1982 Mar;92(3):822–832. doi: 10.1083/jcb.92.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya K., Ui M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin Chim Acta. 1966 Sep;14(3):361–366. doi: 10.1016/0009-8981(66)90114-8. [DOI] [PubMed] [Google Scholar]
- Ito A., Palade G. E. Presence of NADPH-cytochrome P-450 reductase in rat liver Golgi membranes. Evidence obtained by immunoadsorption method. J Cell Biol. 1978 Nov;79(2 Pt 1):590–597. doi: 10.1083/jcb.79.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremmer T., Wisher M. H., Evans W. H. The lipid composition of plasma membrane subfractions originating from the three major functional domains of the rat hepatocyte cell surface. Biochim Biophys Acta. 1976 Dec 14;455(3):655–664. doi: 10.1016/0005-2736(76)90039-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane L. C. A simple method for stabilizing protein-sulfhydryl groups during SDS-gel electrophoresis. Anal Biochem. 1978 Jun 1;86(2):655–664. doi: 10.1016/0003-2697(78)90792-3. [DOI] [PubMed] [Google Scholar]
- Luzio J. P., Newby A. C., Hales C. N. A rapid immunological procedure for the isolation of hormonally sensitive rat fat-cell plasma membrane. Biochem J. 1976 Jan 15;154(1):11–21. doi: 10.1042/bj1540011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G. A., Docherty K., Hales C. N. Sugar transport in rat liver lysosomes. Direct demonstration by using labelled sugars. Biochem J. 1983 Apr 15;212(1):211–218. doi: 10.1042/bj2120211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. S. Endocytosis, membrane recycling and Fc receptor function. Ciba Found Symp. 1982;(92):35–58. [PubMed] [Google Scholar]
- Mellman I. S., Steinman R. M., Unkeless J. C., Cohn Z. A. Selective iodination and polypeptide composition of pinocytic vesicles. J Cell Biol. 1980 Sep;86(3):712–722. doi: 10.1083/jcb.86.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merion M., Sly W. S. The role of intermediate vesicles in the adsorptive endocytosis and transport of ligand to lysosomes by human fibroblasts. J Cell Biol. 1983 Mar;96(3):644–650. doi: 10.1083/jcb.96.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C., Luzio J. P., Hales C. N. The properties and extracellular location of 5'-nucleotidase of the rat fat-cell plasma membrane. Biochem J. 1975 Mar;146(3):625–633. doi: 10.1042/bj1460625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottosen P. D., Courtoy P. J., Farquhar M. G. Pathways followed by membrane recovered from the surface of plasma cells and myeloma cells. J Exp Med. 1980 Jul 1;152(1):1–19. doi: 10.1084/jem.152.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. E. Problems in intracellular membrane traffic. Ciba Found Symp. 1982;(92):1–14. doi: 10.1002/9780470720745.ch1. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Bretscher M. S. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. doi: 10.1146/annurev.bi.50.070181.000505. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., Exton J. H., Johnson R. A., Park C. R. Effects of glucagon on cyclic AMP and carbohydrate metabolism in livers from diabetic rats. Biochim Biophys Acta. 1974 Mar 20;343(1):250–267. doi: 10.1016/0304-4165(74)90258-x. [DOI] [PubMed] [Google Scholar]
- Quintart J., Courtoy P. J., Limet J. N., Baudhuin P. Galactose-specific endocytosis in rat liver. Biochemical and morphological characterization of a low-density compartment isolated from hepatocytes. Eur J Biochem. 1983 Mar 1;131(1):105–112. doi: 10.1111/j.1432-1033.1983.tb07236.x. [DOI] [PubMed] [Google Scholar]
- Reijngoud D. J., Tager J. M. The permeability properties of the lysosomal membrane. Biochim Biophys Acta. 1977 Nov 14;472(3-4):419–449. doi: 10.1016/0304-4157(77)90005-3. [DOI] [PubMed] [Google Scholar]
- Schneider Y. J., Tulkens P., de Duve C., Trouet A. Fate of plasma membrane during endocytosis. II. Evidence for recycling (shuttle) of plasma membrane constituents. J Cell Biol. 1979 Aug;82(2):466–474. doi: 10.1083/jcb.82.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shur B. D., Roth S. Cell surface glycosyltransferases. Biochim Biophys Acta. 1975 Dec 29;415(4):473–512. doi: 10.1016/0304-4157(75)90007-6. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Burke B., Pitt T., Siddle K., Luzio J. P. Localisation of 5'-nucleotidase in a rat liver cell line using a monoclonal antibody and indirect immunofluorescent labelling. Exp Cell Res. 1983 Mar;144(1):39–46. doi: 10.1016/0014-4827(83)90439-1. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Edwards M. R., Luzio J. P. Subcellular distribution and movement of 5'-nucleotidase in rat cells. Biochem J. 1980 Jan 15;186(1):59–69. doi: 10.1042/bj1860059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer C. J., Ashwell G. Studies on a mammalian hepatic binding protein specific for asialoglycoproteins. Evidence for receptor recycling in isolated rat hepatocytes. J Biol Chem. 1980 Apr 10;255(7):3008–3013. [PubMed] [Google Scholar]
- Thilo L., Vogel G. Kinetics of membrane internalization and recycling during pinocytosis in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1015–1019. doi: 10.1073/pnas.77.2.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwood S. A., Luzio J. P., Flockhart D. A., Siddle K. Investigation of the subcellular distribution of cyclic-AMP phosphodiesterase in rat hepatocytes, using a rapid immunological procedure for the isolation of plasma membrane. Biochim Biophys Acta. 1979 Apr 3;583(4):454–466. doi: 10.1016/0304-4165(79)90062-x. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Schneider Y. J., Pierre B., Baudhuin P., Trouet A. Evidence for a continual exchange of 5'-nucleotidase between the cell surface and cytoplasmic membranes in cultured rat fibroblasts. Cell. 1982 Jan;28(1):61–70. doi: 10.1016/0092-8674(82)90375-0. [DOI] [PubMed] [Google Scholar]
- Zambrano F., Fleischer S., Fleischer B. Lipid composition of the Golgi apparatus of rat kidney and liver in comparison with other subcellular organelles. Biochim Biophys Acta. 1975 Mar 24;380(3):357–369. doi: 10.1016/0005-2760(75)90104-6. [DOI] [PubMed] [Google Scholar]