Abstract
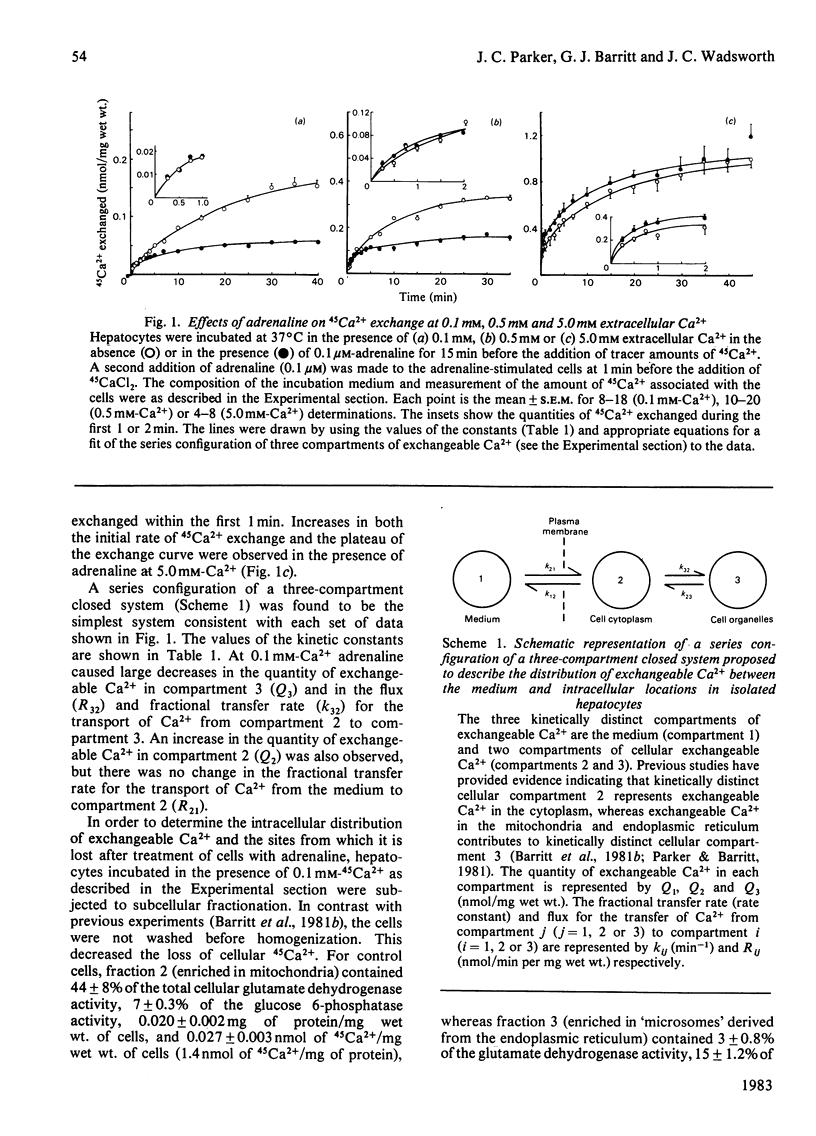
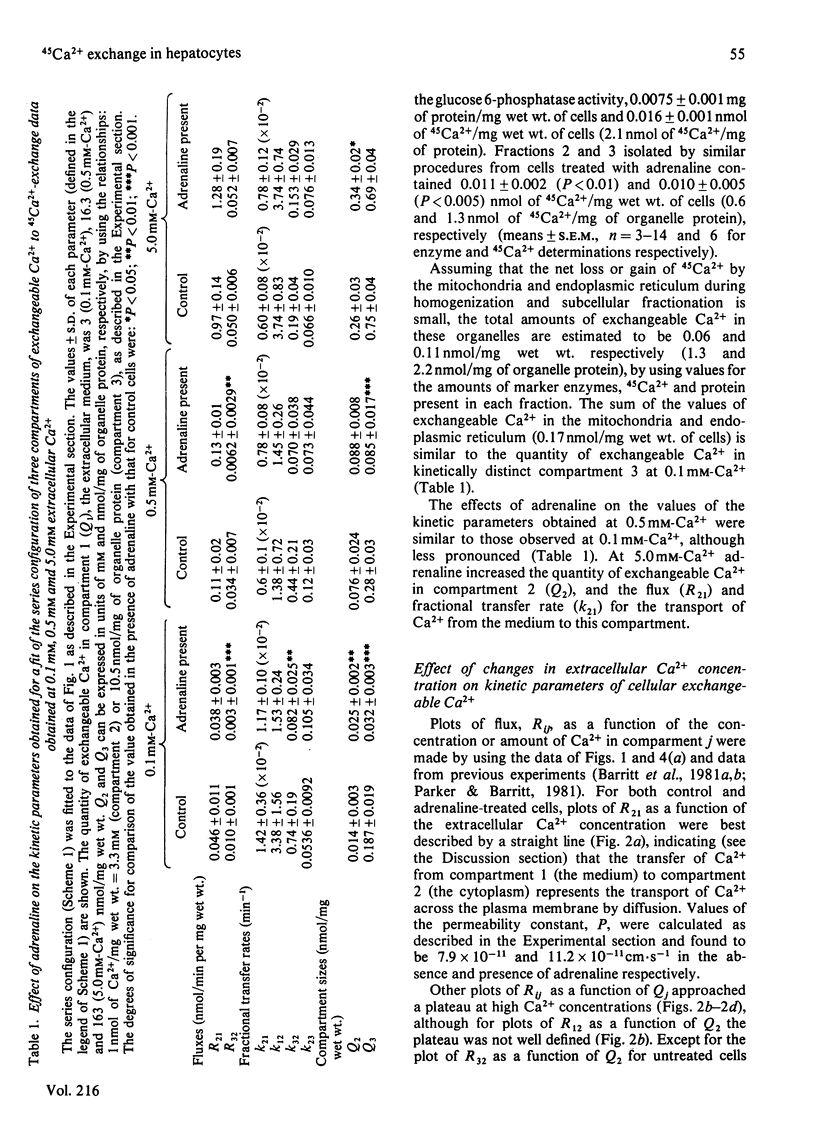
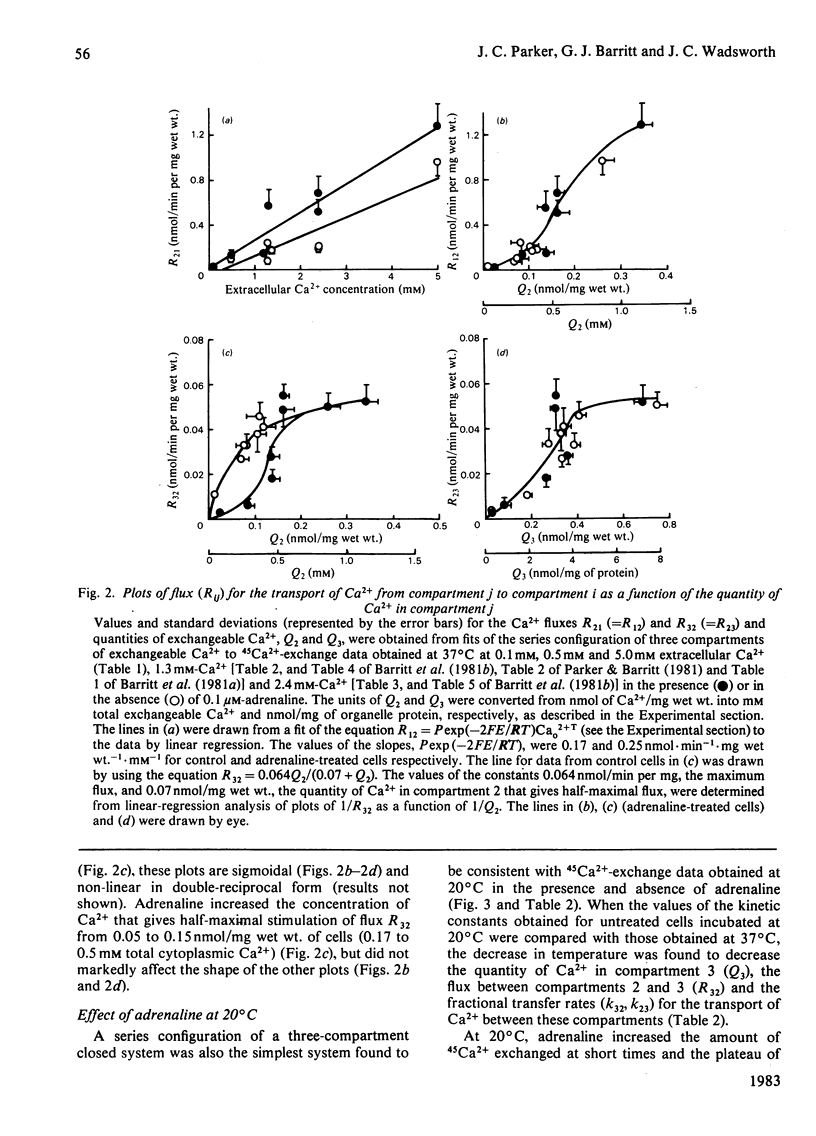
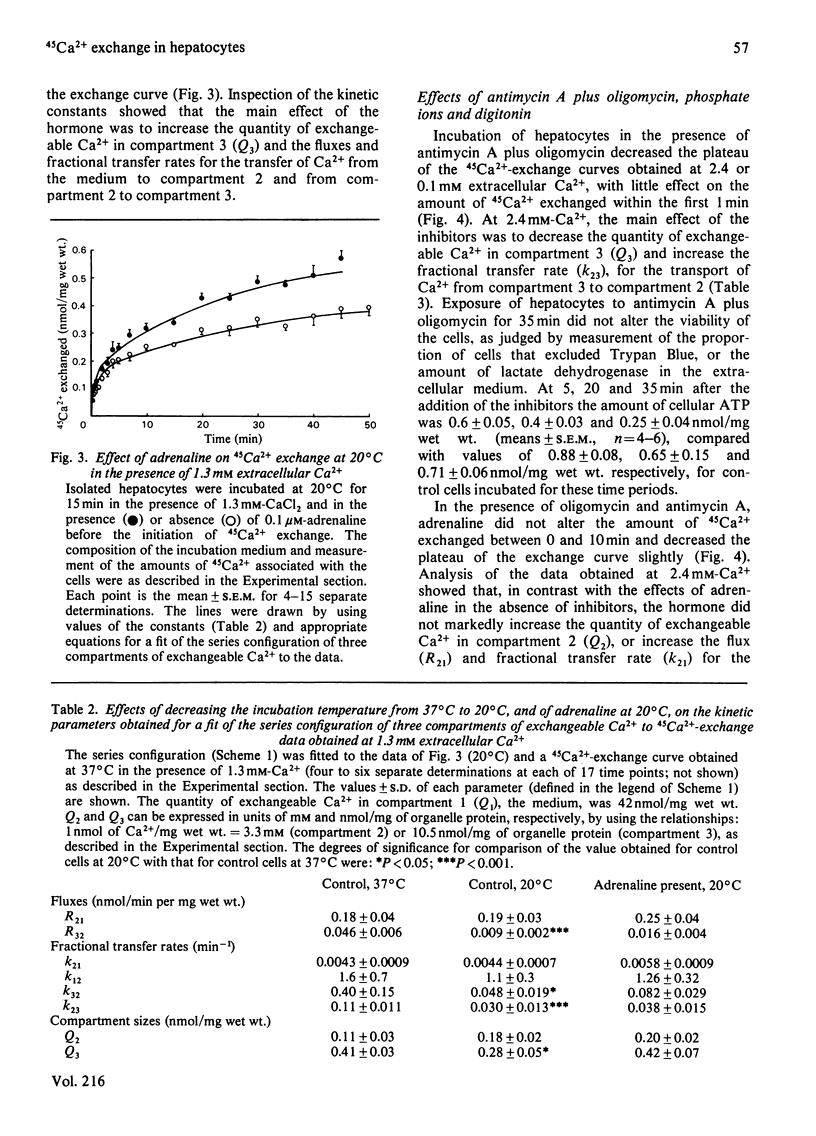
The effects of adrenaline on 45Ca2+-exchange curves for isolated hepatocytes incubated under various steady-state conditions were investigated. Kinetic analysis showed that the simplest compartment configuration consistent with each set of data was a series configuration of a three-compartment closed system comprising compartment 1 (C1), the extracellular medium, and two kinetically distinct compartments of cellular exchangeable Ca2+, C2 and C3 (C1 = C2 = C3). Subcellular fractionation of hepatocytes labelled with 45Ca2+ at 0.1 mM-Ca2+ indicated that C3 includes exchangeable Ca2+ in the mitochondria and endoplasmic reticulum. The following results were obtained from experiments conducted at 37 degrees C at five different extracellular Ca2+ concentrations. For both untreated and adrenaline-treated cells, plots of the flux from C1 to C2 as a function of the extracellular Ca2+ concentration were best described by straight lines consistent with Ca2+ influx across the plasma membrane being a diffusion process. Adrenaline increased the value of the permeability constant for Ca2+ influx by 40%. For untreated cells, plots of the flux between C2 and C3 as a function of the concentrations of Ca2+ in these compartments approached a plateau at high Ca2+ concentrations. Adrenaline caused a 3-fold increase in the concentration of Ca2+ that gives half-maximal rate of Ca2+ transport from C2 to C3. At 1.3 mM extracellular Ca2+, a decrease in incubation temperature from 37 degrees C to 20 degrees C decreased the quantity of Ca2+ in C3 and the flux and fractional transfer rates for the transport of Ca2+ between C2 and C3. At 20 degrees C adrenaline increased the quantity of Ca2+ in C3 and the fractional transfer rates for the transfer of Ca2+ from C1 to C2, and from C2 to C3. At 37 degrees C and 2.4 mM extracellular Ca2+, antimycin A plus oligomycin decreased the quantity of Ca2+ in C3 and increased the fractional transfer rate for the transport of Ca2+ from C3 to C2. In the presence of antimycin A and oligomycin, adrenaline did not increase the quantity of Ca2+ in C2 or the flux and fractional transfer rate for the transport of Ca2+ from C1 to C2, whereas these parameters were increased in the absence of the inhibitors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affolter H., Carafoli E. Hyperbolic kinetics of the electrophoretic carrier of Ca2+ uptake in liver mitochondria. Eur J Biochem. 1981 Sep;119(1):199–201. doi: 10.1111/j.1432-1033.1981.tb05594.x. [DOI] [PubMed] [Google Scholar]
- Althaus-Salzmann M., Carafoli E., Jakob A. Ca2+, K+ redistributions and alpha-adrenergic activation of glycogenolysis in perfused rat livers. Eur J Biochem. 1980 May;106(1):241–248. doi: 10.1111/j.1432-1033.1980.tb06015.x. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F. D., Blackmore P. F., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Studies on role of calcium in alpha-adrenergic activation of phosphorylase. J Biol Chem. 1977 Apr 25;252(8):2662–2669. [PubMed] [Google Scholar]
- Babcock D. F., Chen J. L., Yip B. P., Lardy H. A. Evidence for mitochondrial localization of the hormone-responsive pool of Ca2+ in isolated hepatocytes. J Biol Chem. 1979 Sep 10;254(17):8117–8120. [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature. 1978 Dec 7;276(5688):620–622. doi: 10.1038/276620a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barritt G. J. Analysis of the effects of adrenaline and glucagon on calcium ion movement in liver cells by using a simple compartmental model [proceedings]. Biochem Soc Trans. 1980 Feb;8(1):144–145. doi: 10.1042/bst0080144. [DOI] [PubMed] [Google Scholar]
- Barritt G. J., Dalton K. A., Whiting J. A. Evidence that phosphatidic acid stimulates the uptake of calcium by liver cells but not calcium release from mitochondria. FEBS Lett. 1981 Mar 23;125(2):137–140. doi: 10.1016/0014-5793(81)80703-x. [DOI] [PubMed] [Google Scholar]
- Barritt G. J. Evidence for two compartments of exchangeable calcium in isolated rat liver mitochondria obtained using a 45Ca exchange technique in the presence of magnesium, phosphate, and ATPase at 37 degrees C. J Membr Biol. 1981;62(1-2):53–63. doi: 10.1007/BF01870199. [DOI] [PubMed] [Google Scholar]
- Barritt G. J., Parker J. C., Wadsworth J. C. A kinetic analysis of the effects of adrenaline on calcium distribution in isolated rat liver parenchymal cells. J Physiol. 1981 Mar;312:29–55. doi: 10.1113/jphysiol.1981.sp013614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Grivell A. R., Wallace P. G. Energy-dependent regulation of the steady-state concentrations of the components of the lactate dehydrogenase reaction in liver. FEBS Lett. 1980 Oct 6;119(2):317–322. doi: 10.1016/0014-5793(80)80280-8. [DOI] [PubMed] [Google Scholar]
- Berthon B., Poggioli J., Capiod T., Claret M. Effect of the alpha-agonist noradrenaline on total and 45Ca2+ movements in mitochondria of rat liver cells. Biochem J. 1981 Oct 15;200(1):177–180. doi: 10.1042/bj2000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black B. L., Jarett L., McDonald J. M. The regulation of endoplasmic reticulum calcium uptake of adipocytes by cytoplasmic calcium. J Biol Chem. 1981 Jan 10;256(1):322–329. [PubMed] [Google Scholar]
- Blackmore P. F., Dehaye J. P., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. The role of mitochondrial calcium release in alpha-adrenergic activation of phosphorylase in perfused rat liver. J Biol Chem. 1979 Aug 10;254(15):6945–6950. [PubMed] [Google Scholar]
- Blackmore P. F., Hughes B. P., Shuman E. A., Exton J. H. alpha-Adrenergic activation of phosphorylase in liver cells involves mobilization of intracellular calcium without influx of extracellular calcium. J Biol Chem. 1982 Jan 10;257(1):190–197. [PubMed] [Google Scholar]
- Borle A. B. Kinetic analysis of calcium movements in cell culture. V. Intracellular calcium distribution in kidney cells. J Membr Biol. 1972;10(1):45–66. doi: 10.1007/BF01867847. [DOI] [PubMed] [Google Scholar]
- Boxenbaum H. G., Riegelman S., Elashoff R. M. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974 Apr;2(2):123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- Brattin W. J., Jr, Waller R. L., Recknagel R. O. Analysis of microsomal calcium sequestration by steady state isotope exchange. Enzyme kinetics and role of membrane permeability. J Biol Chem. 1982 Sep 10;257(17):10044–10051. [PubMed] [Google Scholar]
- Brinley F. J., Jr, Tiffert T., Scarpa A., Mullins L. J. Intracellular calcium buffering capacity in isolated squid axons. J Gen Physiol. 1977 Sep;70(3):355–384. doi: 10.1085/jgp.70.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. In vivo effect of uncoupling agents on the incorporation of calcium and strontium into mitochondria and other subcellular fractions of rat liver. J Gen Physiol. 1967 Aug;50(7):1849–1864. doi: 10.1085/jgp.50.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Babcock D. F., Lardy H. A. Norepinephrine, vasopressin, glucagon, and A23187 induce efflux of calcium from an exchangeable pool in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1978 May;75(5):2234–2238. doi: 10.1073/pnas.75.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret-Berthon B., Claret M., Mazet J. L. Fluxes and distribution of calcium in rat liver cells: kinetic analysis and identification of pools. J Physiol. 1977 Nov;272(3):529–552. doi: 10.1113/jphysiol.1977.sp012058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M., Mazet J. L. Ionic fluxes and permeabilities of cell membranes in rat liver. J Physiol. 1972 Jun;223(2):279–295. doi: 10.1113/jphysiol.1972.sp009847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Physiological role of ATP-driven calcium pump in squid axon. Nature. 1979 Mar 15;278(5701):271–273. doi: 10.1038/278271a0. [DOI] [PubMed] [Google Scholar]
- DiPolo R. Calcium influx in internally dialyzed squid giant axons. J Gen Physiol. 1979 Jan;73(1):91–113. doi: 10.1085/jgp.73.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulski K., Carafoli E. Ca2+ transporting activity of membrane fractions isolated from the post-mitochondrial supernatant of rat liver. Cell Calcium. 1982 Aug;3(3):263–281. doi: 10.1016/0143-4160(82)90005-7. [DOI] [PubMed] [Google Scholar]
- Ferreira H. G., Lew V. L. Use of ionophore A23187 to measure cytoplasmic Ca buffering and activation of the Ca pump by internal Ca. Nature. 1976 Jan 1;259(5538):47–49. doi: 10.1038/259047a0. [DOI] [PubMed] [Google Scholar]
- Foden S., Randle P. J. Calcium metabolism in rat hepatocytes. Biochem J. 1978 Mar 15;170(3):615–625. doi: 10.1042/bj1700615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett H. M., Kemp R. B. (Ca-2+ + Mg-2+)-Activated ATPase in the plasma membrane mouse liver cells. Biochim Biophys Acta. 1975 Apr 8;382(4):526–533. doi: 10.1016/0005-2736(75)90219-9. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett D. G., Jenkinson D. H. Effects of noradrenaline on potassium reflux, membrane potential and electrolyte levels in tissue slices prepared from guinea-pig liver. J Physiol. 1972 Sep;225(3):721–750. doi: 10.1113/jphysiol.1972.sp009966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen R., Miyamoto H., Racker E. Ca2+ translocation in Ehrlich ascites tumor cells. J Membr Biol. 1979 Sep 14;49(4):309–324. doi: 10.1007/BF01868989. [DOI] [PubMed] [Google Scholar]
- Hope-Gill H. F., Nanda V. Stimulation of calcium ATPase by insulin, glucagon, cyclic AMP and cyclic GMP in Triton X-100 extracts of purified rat liver plasma membrane. Horm Metab Res. 1979 Dec;11(12):698–700. doi: 10.1055/s-0028-1095813. [DOI] [PubMed] [Google Scholar]
- Iwasa Y., Iwasa Y., Higashi K., Matsui K., Miyamoto E. Demonstration of a high affinity Ca2+ ATPase in rat liver plasma membranes. Biochem Biophys Res Commun. 1982 Mar 30;105(2):488–494. doi: 10.1016/0006-291x(82)91461-9. [DOI] [PubMed] [Google Scholar]
- Jenkinson D. H., Koller K. Interactions between the effects of alpha- and beta-adrenoceptor agonists and adenine nucleotides on the membrane potential of cells in guinea-pig liver slices. Br J Pharmacol. 1977 Jan;59(1):163–175. doi: 10.1111/j.1476-5381.1977.tb06991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Coll K. E., Cooper R. H., Marks J. S., Williamson J. R. Mechanisms underlying calcium homeostasis in isolated hepatocytes. J Biol Chem. 1983 Jan 25;258(2):731–741. [PubMed] [Google Scholar]
- Keppens S., Vandenheede J. R., De Wulf H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim Biophys Acta. 1977 Feb 28;496(2):448–457. doi: 10.1016/0304-4165(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Kimura S., Kugai N., Tada R., Kojima I., Abe K., Ogata E. Sources of calcium mobilized by alpha-adrenergic stimulation in perfused rat liver. Horm Metab Res. 1982 Mar;14(3):133–138. doi: 10.1055/s-2007-1018947. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Adam G. Regulation of ion permeabilities of isolated rat liver cells by external calcium concentration and temperature. J Membr Biol. 1976 Mar 18;26(2-3):121–151. doi: 10.1007/BF01868870. [DOI] [PubMed] [Google Scholar]
- Kondo S., Schulz I. Ca++ fluxes in isolated cells of rat pancreas. effect of secretagogues and different Ca++ concentrations. J Membr Biol. 1976 Oct 20;29(1-2):185–203. doi: 10.1007/BF01868959. [DOI] [PubMed] [Google Scholar]
- Krack G., Gravier O., Roberfroid M., Mercier M. Subcellular fractionation of isolated rat hepatocytes. A comparison with liver homogenate. Biochim Biophys Acta. 1980 Nov 3;632(4):619–629. doi: 10.1016/0304-4165(80)90338-4. [DOI] [PubMed] [Google Scholar]
- Kraus-Friedmann N., Biber J., Murer H., Carafoli E. Calcium uptake in isolated hepatic plasma-membrane vesicles. Eur J Biochem. 1982 Dec;129(1):7–12. doi: 10.1111/j.1432-1033.1982.tb07014.x. [DOI] [PubMed] [Google Scholar]
- Krell H., Baur H., Pfaff E. Transient 45Ca uptake and release in isolated rat-liver cells during recovery from deenergized states. Eur J Biochem. 1979 Nov;101(2):349–364. doi: 10.1111/j.1432-1033.1979.tb19727.x. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Nudd L. M. Addition and kinetic characterization of mitochondrial calcium in myocardial tissue culture. Am J Physiol. 1980 Dec;239(6):H769–H774. doi: 10.1152/ajpheart.1980.239.6.H769. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Lotersztajn S., Hanoune J., Pecker F. A high affinity calcium-stimulated magnesium-dependent ATPase in rat liver plasma membranes. Dependence of an endogenous protein activator distinct from calmodulin. J Biol Chem. 1981 Nov 10;256(21):11209–11215. [PubMed] [Google Scholar]
- Muallem S., Karlish S. J. Regulation of the Ca2+-pump by calmodulin in intact cells. Biochim Biophys Acta. 1982 May 7;687(2):329–332. doi: 10.1016/0005-2736(82)90563-6. [DOI] [PubMed] [Google Scholar]
- Murphy E., Coll K., Rich T. L., Williamson J. R. Hormonal effects on calcium homeostasis in isolated hepatocytes. J Biol Chem. 1980 Jul 25;255(14):6600–6608. [PubMed] [Google Scholar]
- Parker J. C., Barritt G. J. Evidence that lanthanum ions stimulate calcium inflow to isolated hepatocytes. Biochem J. 1981 Oct 15;200(1):109–114. doi: 10.1042/bj2000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli J., Berthon B., Claret M. Calcium movements in in situ mitochondria following activation of alpha-adrenergic receptors in rat liver cells. FEBS Lett. 1980 Jun 30;115(2):243–246. doi: 10.1016/0014-5793(80)81178-1. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Weiss S. J., McKinney J. S., Putney J. W., Jr Effects of antimycin A on receptor-activated calcium mobilization and phosphoinositide metabolism in rat parotid gland. Mol Pharmacol. 1983 Jan;23(1):71–77. [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. A kinetic study of mitochondrial calcium transport. Eur J Biochem. 1975 Jul 15;55(3):497–504. doi: 10.1111/j.1432-1033.1975.tb02187.x. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Calcium ion fluxes induced by the action of alpha-adrenergic agonists in perfused rat liver. Biochem J. 1982 Dec 15;208(3):619–630. doi: 10.1042/bj2080619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Studies on alpha-adrenergic-induced respiration and glycogenolysis in perfused rat liver. J Biol Chem. 1982 Feb 25;257(4):1906–1912. [PubMed] [Google Scholar]
- Simonsen L. O., Gomme J., Lew V. L. Uniform ionophore A23187 distribution and cytoplasmic calcium buffering in intact human red cells. Biochim Biophys Acta. 1982 Nov 22;692(3):431–440. doi: 10.1016/0005-2736(82)90394-7. [DOI] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]
- Van Bossum G. D., Smith K. P., Beeton P. Role of mitochondria in control of calcium content of liver slices. Nature. 1976 Mar 25;260(5549):335–337. doi: 10.1038/260335a0. [DOI] [PubMed] [Google Scholar]
- Van Rossum G. D. Net movements of calcium and magnesium in slices of rat liver. J Gen Physiol. 1970 Jan;55(1):18–32. doi: 10.1085/jgp.55.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov A., Scarpa A. The initial velocities of calcium uptake by rat liver mitochondria. J Biol Chem. 1973 Aug 10;248(15):5527–5531. [PubMed] [Google Scholar]
- Whiting J. A., Barritt G. J. On the mechanism by which hormones induce the release of Ca2+ from mitochondria in the liver cell. Biochem J. 1982 Jul 15;206(1):121–129. doi: 10.1042/bj2060121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Hoek J. B. Role of calcium in the hormonal regulation of liver metabolism. Biochim Biophys Acta. 1981 Dec 30;639(3-4):243–295. doi: 10.1016/0304-4173(81)90012-4. [DOI] [PubMed] [Google Scholar]


