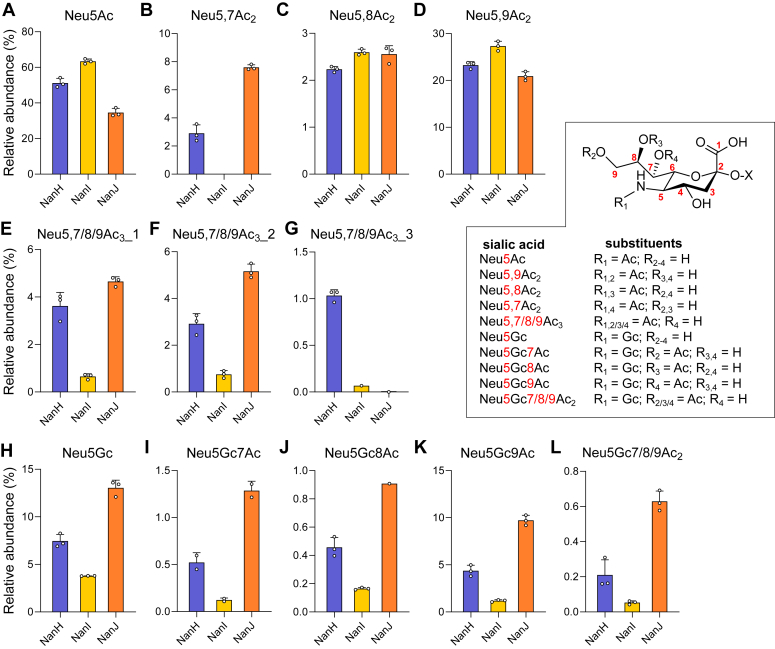
Figure 3.
Assessing the tolerance of C. perfringens sialidases against O-acetylated sialic acids.A–L, release of O-acetylated sialic acid species indicated in the panels by each enzyme. Bovine submaxillary mucin (BSM) was treated with NanIGH33, NanJGH33, and NanHGH33 for 5.5 h after which liberated sialic acids were derivatized and analyzed by HPLC-MS. Activities of each sialidase were normalized based on their rate of hydrolyzing 4MU-Neu5Ac. Three replicates were assessed per sialidase, with error bars representing the standard deviation of the mean. In all instances, MS detector responses for each unique sialic acid were normalized to the total detectible sialic acid pool in each sample. Except for Neu5Ac, Neu5Gc, and Neu5,9Ac2, all assignments are putative albeit Neu5,7Ac2, Neu5,8Ac2 (and their Neu5Gc congeners) are consistent with their previously reported retention times and fragmentation patterns. In E-G, triacetylated substrates at four positions (5 and 7/8/9) were not confidently identified and are thus listed as Neu5,7/8/9_1, _2, and _3. Based on microarray data (Fig. 2), we propose what we have annotated as Neu5,7/8/9Ac3_3 is Neu5,7,9Ac3. Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolylneuraminic acid; HPLC-MS, high-performance liquid chromatography coupled to mass spectrometry.