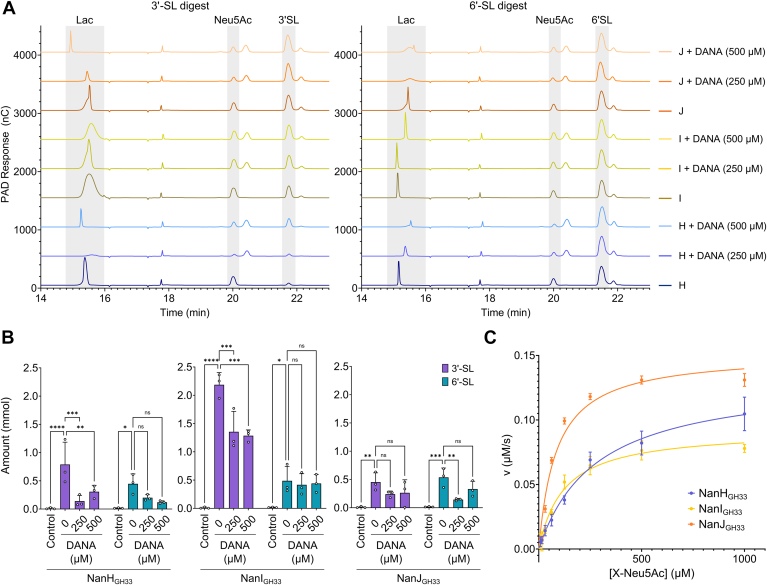
Figure 8.
Enzymatic hydrolysis of sialyllactose and X-Neu5Ac substrates.A, enzymatic digests of 3′-sialyllactose and 6′-sialyllactose substrates by the sialidases were analyzed by HPAEC-PAD in triplicate, with one replicate trace shown. B, released Lac, as analyzed by HPAEC-PAD, was quantified using Chromeleon software (Thermo Fisher Scientific) and the known concentration series of standards as outlined. Amounts of Lac disaccharide are shown as averages of triplicate HPAEC-PAD injections, and error bars represent the standard deviation of the mean. Statistical significance is indicated by asterisks (∗ = p < 0.05, ∗∗ = p < 0.005, ∗∗∗ = p < 0.0005, ∗∗∗∗ = p < 0.0001), as calculated using a 2-way ANOVA. C, the initial rates of X-Neu5Ac substrate hydrolysis by the GH33 domains were obtained and fit to the Michaelis-Menten equation. Data were obtained in triplicate, and error bars represent the standard error of the mean. Neu5Ac, N-acetylneuraminic acid; X-Neu5Ac, 5-bromo-4-chloro-3-indolyl α-D-N-acetylneuraminic acid; HPAEC-PAD, high-performance anion-exchange chromatography with pulsed amperometric detection.