Abstract
Background
Intermittent ambulatory levosimendan administration has been shown in several small randomized controlled trials to benefit patients with advanced heart failure, preventing heart failure rehospitalization and mortality. We aim to investigate the totality of high-quality evidence regarding the efficacy and safety of intermittent levosimendan in advanced heart failure patients.
Methods
Up to September 2023, we systematically reviewed the randomized controlled trials indexed in PubMed, Embase Cochrane, SCOPUS, and Web of Science. We used mean difference (MD) to estimate the continuous outcomes, and risk ratio (RR) for the dichotomous outcomes with a 95% confidence interval (CI), using the random-effects model. Ultimately, a trial sequential analysis was employed to enhance the reliability of our findings and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework for certainty leveling.
Results
Fifteen randomized controlled trials with 1181 patients were included. Intermittent levosimendan was significantly associated with an improved left ventricular ejection fraction compared with placebo (MD 6.39 [95% CI 3.04–9.73], P = 0.002; I2 = 75, P = 0.0005), with cumulative z-score of change after ≤ 1 week passing the monitoring boundaries, favoring the levosimendan, but did not cross the required information size. Additionally, levosimendan reduced the all-cause mortality rate (RR 0.60 [95% CI 0.40–0.90], P = 0.01; I2 = 9, P = 0.36). However, we found no difference between levosimendan and placebo in all-cause rehospitalization rate (RR 0.75 [95% CI 0.46–1.22], P = 0.25; I2 = 70, P = 0.04), event-free survival rate (RR 0.97 [95% CI 0.72–1.30], P = 0.84; I2 = 63, P = 0.03), or any adverse event (RR 1 [95% CI 0.73–1.37], P = 1.00, I2 = 0%, P = 0.70).
Conclusion
In patients with advanced heart failure, intermittent levosimendan significantly improved left ventricular ejection fraction, brain natriuretic peptide values, and all-cause mortality rate. Levosimendan use is not associated with a change in rehospitalization or event-free survival.
Registration
PROSPERO identifier number (CRD42023487838).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40256-024-00675-z.
Key Points
| Levosimendan significantly enhanced heart performance and lowered death rates in patients with severe heart failure. |
| Despite its benefits for heart function and survival, levosimendan did not reduce the frequency of hospital readmissions or overall survival without events. |
| The use of levosimendan did not lead to an increase in adverse side effects compared with placebo. |
Introduction
Heart failure is a severe pandemic affecting an estimated 64 million individuals in 2017 [1]. The prevalence rate of heart failure ranges from 1 to 3%, and the lifetime risk of heart failure has increased to one in four individuals. The 1-year mortality risk associated with index diagnosis is 15–30%. Mortality risk is increased dramatically following acute heart failure hospitalization, at approximately 11% over the next 90 days post-discharge [2]. Moreover, the projected increase in heart failure prevalence from 2012 to 2030 is 46%, and the total cost is expected to rise from $30.7 billion to $69.8 billion (US dollars) between 2012 and 2030 [3, 4]. The current guideline-directed medical therapy (GDMT) aims to reduce heart failure mortality and hospitalizations [5]. However, targeted interventions decreasing morbidity and mortality during the vulnerable post-discharge period are still lacking [6].
Levosimendan is an inodilator calcium-sensitizing agent with the following effects: (1) inotropic effect via increasing troponin C sensitivity to calcium without increasing intracellular calcium, (2) vasodilator effect via opening potassium channels in vasculature smooth muscles, and (3) cardio-protection against ischemia via the activation of potassium channels in cardiac mitochondria. It is also postulated that levosimendan possesses cardioprotective properties as a calcium sensitizer offering a neutral effect on myocardial oxygen consumption and modulating a favorable oxidative balance [7]. These properties may lead to protection against arrhythmias and cardiac remodeling [8], as opposed to inotropic agents that increase myocardial oxygen demand and promote arrhythmias [9].
The hemodynamic effects of intravenous (IV) levosimendan encompass a dose-dependent stroke volume and cardiac output elevation while cardiac filling pressures are reduced [10]. Finally, the neurohormonal effects of IV levosimendan include a reduction in natriuretic peptides, interleukin-6, and high-sensitivity C-reactive protein (hs-CRP) [11–13].
Levosimendan is available in Europe and South America but is not approved in the USA. IV inotropes (e.g., dobutamine and milrinone) are largely limited to inpatient settings and palliative efforts due to studies suggesting increased mortality and lack of improved clinical outcomes with long-term use [14]. Furthermore, although milrinone may exert favorable hemodynamic effects, short-term use did not improve cumulative days of hospitalization for cardiovascular cause or mortality within 60 days of administration [15]. A great unmet need exists to improve outcomes in high-risk heart failure patients; levosimendan may meet that need. However, evidence is sparse regarding the clinical benefits of intermittent levosimendan use, with several randomized controlled trials (RCTs) investigating its potential effect on advanced heart failure outcomes [6, 11, 13, 16–27].
To thoroughly evaluate the existing evidence, this systematic review and meta-analysis investigates levosimendan's efficacy and safety in advanced heart failure management.
Methods
Registration
The PROSPERO registration ID of this study is (CRD42023487838). We conducted this study following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [28] and the Cochrane Handbook for Systematic Reviews and Meta-Analyses [29].
Data Sources and Search Strategy
A.S.E. and B.A. conducted a comprehensive search across multiple databases including PubMed, SCOPUS, CENTRAL, Web of Science, and Embase, without imposing any restrictions, until September 2023. Further information regarding the search methodology is detailed in Table S1 (see the electronic supplementary material).
Eligibility Criteria
We included RCTs that met our predefined criteria: population (P) consisted of advanced chronic heart failure patients; intervention (I) involved the administration of levosimendan, regardless of the treatment regimen; comparison (C) was standard of care without levosimendan administration or placebo. Outcomes (O) were as follows: our primary outcome was the change in left ventricular ejection fraction (LVEF), while secondary outcomes were event-free survival/time to first hospitalization, change in brain natriuretic peptide (BNP), all-cause rehospitalization, all-cause mortality, hypotension, and any adverse event.
Study Selection
A.M.A., A.R.S., S.E., and M.E. conducted individual screening of titles and abstracts using Covidence. After duplicate removal, the four reviewers independently screened the full texts according to our eligibility criteria. Any conflicts were resolved by consensus.
Data Extraction
A.M.A., S.E., M.E., and A.R.S. independently conducted data extraction from the included trials using Excel sheets. Any discrepancies were resolved through consensus. This sheet encompassed the following: (1) a summary section (including study design, country, total participants, inclusion and exclusion criteria, details of levosimendan prescription, and follow-up period); (2) baseline characteristics (such as gender, age, body mass index, medical history including diabetes, hypertension, coronary artery disease, and clinical parameters); and (3) study outcomes (including event-free survival/time to first event, change in BNP, change in LVEF, all-cause mortality, all-cause rehospitalization, hypotension, and any adverse events). Conflicts were resolved through consensus.
Risk of Bias and Certainty of Evidence
A.M.A., S.E., M.E., and A.R. used ROB-II to assess the quality of the included studies. ROB-II investigates the risk of bias according to five domains (randomization, deviations from intended interventions, missing outcome data, outcome measurement, and selection of the reported result). Any conflict was handled through discussion or by inviting A.S.E. to make a final decision [30]. Furthermore, M.A. applied the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines to appraise the quality of evidence [31, 32].
Statistical Analysis
We used Review Manager (RevMan) software to pool the data of the included trials [33]. We calculated the risk ratio (RR) for the dichotomous outcomes and mean difference (MD) for the continuous outcomes, along with a 95% confidence interval (CI). To handle differences in the study settings and participant demographics, we used a random-effects model to address potential heterogeneity [34]. To determine between-study variance (tau-squared) within the random-effects model, we applied the DerSimonian and Laird method. We assessed heterogeneity using the I2 statistic, which gauges the part of total variability due to heterogeneity instead of random chance. As per the Cochrane Handbook [35], the Chi-squared test was evaluated as significant heterogeneity if the alpha level was less than 0.1, while the I2 test was interpreted as follows: not significant was indicated by 0–40%, moderate heterogeneity was indicated by 30–60%, and substantial heterogeneity was indicated by 50–90% [35]. We carried out a leave-one-out sensitivity analysis to assess the robustness of the pooled results. Also, leave-one-out analysis is useful to investigate the influence of each study on the overall effect-size estimate and to identify influential studies. If the P value was less than 0.05, the total effect size was regarded as statistically significant. Furthermore, considering the relatively small number of studies included in some outcomes and to improve the reliability of our results, we implemented a trial sequential analysis (TSA) to balance type I and type II errors and provide an estimate of when the effect size would be substantial enough to withstand the impact of additional studies [36, 37].
Results
Search Results and Study Selection
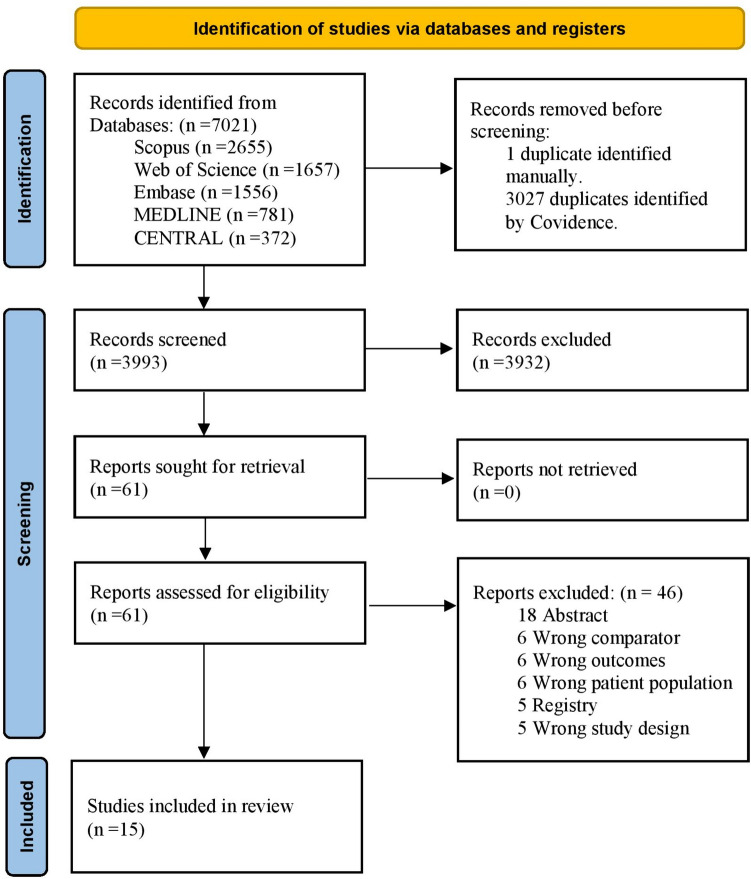
Initially, 7021 records were identified after extensive searches across databases. Following removal of duplicate entries, 3993 studies were deemed eligible for title and abstract screening. Among these, 3932 studies were excluded as they did not align with our research objectives. Subsequently, 61 articles underwent full-text screening. Ultimately, we included 15 eligible RCTs [6, 11, 13, 16–27]. Figure 1 illustrates the PRISMA flow diagram.
Fig. 1.
PRISMA flow chart of the screening process. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analysis
Baseline Characteristics
Fifteen eligible RCTs involving 1181 patients were included [6, 11, 13, 16–27]. The levosimendan treatment regimen varied significantly among the trials, as outlined in Table 1. Treatment duration and follow-up periods ranged from 24 h to 12 months. Approximately 76% of the enrolled patients were males, with mean ages spanning from 50.2 to 80.8 years. Also, about 51.4% of the included patients had a history of hypertension, and 18.5% had a history of coronary disease, with a mean LVEF range from 20 to 33.4%. Additional baseline characteristics of the enrolled participants are provided in Table 2. Moreover, Table S2 in the electronic supplementary material provides detailed inclusion and exclusion criteria of the trials.
Table 1.
Summary of the included studies
| Study ID | Country | Study design | Sample size | Levosimendan dosage | Follow-up duration | ||
|---|---|---|---|---|---|---|---|
| Bolus dose | Maintenance dose | Treatment duration | |||||
| Pölzl et al. 2023 [6] | Austria | RCT | 148 | No bolus dose | 6-h infusion: 0.2 µg/kg/min over 6 h every 2 weeks for 12 weeks | 12 weeks | 180 ± 14 days |
| 24-h infusion: 0.1 µg/kg/min over 24 h every 3 weeks for 12 weeks | |||||||
| García-González et al. 2021 [16] | Spain | RCT | 97 | No bolus dose | 0.1 µg/kg/min, IV over 24 h once every 30 days | 12 months | 12 months |
| Cui et al. 2020 [26] | China | RCT | 49 | 12 µg/kg, IV for 10 min | 0.1 µg/kg/min, IV over 24 h | 24 h | 1 month |
| Comín-Colet et al. 2018 [25] | Spain | RCT | 69 | No bolus dose | 0.2 µg/kg/min, IV over 6 h | 12 weeks | 25 weeks |
| Zhang et al. 2015 [24] | China | RCT | 42 | 12 µg/kg, IV for 10 min | 0.1 µg/kg/min, IV over an hour. Then, 0.2 µg/kg/min, IV over 23 h | 24 h | 4 weeks |
| Shah et al. 2014 [23] | India | RCT | 50 | 12.5 mg/mL, IV | 200 µg/kg dose in 50 mL of normal saline at the rate of 2 mL/h for 24 h, IV | 24 h | 2 months |
| Altenberger et al. 2014 [27] | Austria, Greece, and Germany | RCT | 120 | No bolus dose |
0.2 µg/kg/min, IV over 6 h Four cycles of levosimendan at 2-week intervals |
6 weeks | 18 weeks |
| Llorens et al. 2012 [19] | Spain | RCT | 45 |
In patients with SBP > 120 mmHg: 6 µg/kg, IV for 10 min In patients with SBP = 90: 120 mmHg: No bolus dose |
0.1 µg/kg/min, IV over 24 h In patients who experienced a decline of SBP below 90 mmHg, the dose was reduced by half, and nitroglycerin tapered gradually to withdrawal if necessary |
24 h | 6 months |
| Kurt et al. 2010 [17] | Turkey | RCT | 60 | 12 µg/kg, IV for 10 min | 0.1 µg/kg/min, IV over 24 h | 24 h | NA |
| Nieminen et al. 2008 [21] | Finland, Estonia, Latvia, Lithuania, and Russia | RCT | 307 | No bolus dose |
LS-1: 1-mg capsule once daily LS-2: 1-mg capsule twice daily |
180 days | 180 days |
| Mavrogeni et al. 2007 [20] | Greece | RCT | 50 | 6 µg/kg, IV for 10 min | 0.1 µg/kg/min, IV over an hour. Then, 0.2 µg/kg/min, IV over 23 h | 8 months | 6 months |
| Parissis et al. 2007 [22] | Greece | RCT | 63 | No bolus dose | 0.1 µg/kg/min, IV over 24 h | 24 h | NA |
| Lilleberg et al. 2007 [18] | Finland | RCT | 22 | 12 µg/kg, IV for 10 min | 0.1 µg/kg/min, IV over an hour. Then, 0.2 µg/kg/min, IV over 23 h | 25 h | 14 days |
| Parissis et al. 2006 [11] | Greece | RCT | 25 | 6 µg/kg, IV for 10 min | 0.1 µg/kg/min, IV over an hour. Then, 0.4 µg/kg/min, IV over 23 h | 15 weeks (5 repetitive infusions) | 1 month |
| Parissis et al. 2005 [13] | Greece | RCT | 34 | 6 µg/kg, IV for 10 min | 0.1 µg/kg/min, IV over an hour. Then, 0.4 µg/kg/min, IV over 23 h | 24 h | 5 months |
IV intravenous, LS-1 1-mg capsule of levosimendan once daily group, LS-2 1-mg capsule of levosimendan twice daily group, NA not available, RCT randomized controlled trial, SBP systolic blood pressure
Table 2.
Baseline characteristics of the included studies
| Study ID | General characteristics | Medical history | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (male) | Age (years) | BMI | Hypertension | Diabetes | Coronary disease | |||||||
| Levosimendan | Placebo | Levosimendan | Placebo | Levosimendan | Placebo | Levosimendan | Placebo | Levosimendan | Placebo | Levosimendan | Placebo | |
| Pölzl et al. 2023 [6] | 72 (77.5) | 41 (78.8) | 69.3 ± 9.6 | 67.8 ± 10.1 | 26.9 ± 4.9 | 29.6 ± 5.8 | 59 (63.4) | 33 (63.5) | 38 (40.9) | 26 (50.0) | NA | NA |
| Cui et al. 2020 [26] | 22 (85) | 19 (83) | 50.2 ± 13.4 | 54.4 ± 13.2 | NA | NA | 9 (34.6) | 11 (47.8) | 5 (19.2) | 7 (30.4) | 4 (15.4) | 9 (39.1) |
| Shah et al. 2014 [23] | 15 (60) | 16 (64.0) | 59.9 ± 8.8 | 61.3 ± 7.6 | NA | NA | 18 (72) | 20 (80) | 16 (64.0) | 13 (52.0) | NA | NA |
| Zhang et al. 2015 [24] | 10 (48) | 10 (48) | 74.8 ± 4.5 | 74.5 ± 4.3 | NA | NA | 8 (38) | 7 (33) | 5 (25) | 6 (29) | 8 (38) | 8 (38) |
| Altenberger et al. 2014 [27] | 50 (79) | 45 (79%) | 69.5 ± 11.5 | 69.5 ± 10.5 | NA | NA | 36 (57.1) | 37 (64.3) | 22 (34.9) | 24 (42.9) | 39 (61.9) | 32 (57.1) |
| Kurt et al. 2010 [17] | 17 (54.8) | 17 (58.6) | 63.3 ± 11.9 | 64.87 ± 10.4 | NA | NA | 9 (29) | 12 (41.4) | 9 (29) | 6 (20.7) | NA | NA |
| Nieminen et al. 2008 [21] |
LS-1: 77 (75) LS-2: 84 (82) |
86 (84) |
LS-1: 65 ± 10 LS-2: 62 ± 12 |
63 ± 1 |
LS-1: 28 ± 5.2 LS-2: 29 ± 5.4 |
30 ± 5.7 |
LS-1: 71 (70) LS-2: 64 (62) |
63 (62) |
LS-1: 27 (27) LS-2: 24 (23) |
23 (23) |
LS-1: 61 (60) LS-2: 64 (62) |
64 (63) |
| García-González et al. 2021 [16] | 62 (88.6) | 20 (74.1) | 68.1 ± 11.1 | 71.33 ± 9 | NA | NA | 47 (67.1) | 17 (63) | 36 (51.43) | 14 (51.9) | 33 (47.1) | 13 (48.2) |
| Mavrogeni et al. 2007 [20] | 20 (80) | 20 (80) | 62 ± 20 | 61 ± 19 | NA | NA | NA | NA | NA | NA | NA | NA |
| Parissis et al. 2007 [22] | 35 (83.3) | 17 (81) | 65 ± 8 | 66 ± 8 | NA | NA | NA | NA | NA | NA | NA | NA |
| Lilleberg et al. 2007 [18] | 11 (100) | 7 (63.6) | 55 ± 9 | 55 ± 8 | NA | NA | NA | NA | NA | NA | 5 | 2 |
| Comín-Colet et al. 2018 [25] | 41 (85.4) | 16 (76) | 68 ± 10 | 63 ± 9 | 27 ± 4 | 27 ± 5 | 32 (67) | 13 (62) | 24 (50) | 11 (52) | NA | NA |
| Parissis et al. 2006 [11] | 16 (94.1) | 7 (87.5) | 67 ± 6 | 70 ± 8 | NA | NA | NA | NA | NA | NA | NA | NA |
| Parissis et al. 2005 [13] | 16 (94.1) | 15 (82.3) | 66 ± 5 | 68 ± 5 | NA | NA | NA | NA | NA | NA | NA | NA |
| Llorens et al. 2012 [19] | 6 (24) | 7 (35) | 80.8 ± 7.9 | 77.6 ± 9.9 | NA | NA | 24 (96) | 17 (85) | 14 (56) | 7 (35) | 5 (20) | 4 (20) |
| Study ID | Clinical parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LVEF | Heart rate (bpm) | SBP (mm Hg) | DBP (mm Hg) | NYHA functional class | NT-proBNP (pg/mL) | |||||||
| Levosimendan | Placebo | Levosimendan | Placebo | Levosimendan | Placebo | Levosimendan | Placebo | Levosimendan | Placebo | Levosimendan | Placebo | |
| Pölzl et al. 2023 [6] | 24 ± 5 | 24 ± 5 | 69 ± 20.7 | 66.4 ± 22.4 | 101.3 ± 28.7 | 101.4 ± 29.1 | NA | NA |
II: 4 (4.3) IIa: 59 (63.4) IIIb: 26 (28.0) IV: 4 (4.3) |
II: 5 (9.6) IIIa: 29 (55.8) IIIb: 17 (32.7) IV: 1 (1.9) |
5330.3 ± 5106.3 | 5290.3 ± 4511.4 |
| García-González et al. 2021 [16] | 24.6 ± 7.9 | 26 ± 9.9 | NA | NA | NA | NA | NA | NA |
III: 64 (91.43) IV: 5 (7.14) |
III: 25 (92.59) IV: 1 (3.7) |
7963 ± 1564.3 | 14232 ± 5373 |
| Cui et al. 2020 [26] | 30.2 ± 7.2 | 33.4 ± 4.7 | 86.2 ± 13.1 | 82.7 ± 16.6 | 121.9 ± 14.51 | 126.7 ± 24.55 | 80.4 ± 11.91 | 83.7 ± 14.9 |
III: 14 (53.8) IV: 12 (46.2) |
III: 12 (52.2) IV: 11 (47.8) |
4715.6 ± 6881.2 | 4380.4 ± 4350.1 |
| Comín-Colet et al. 2018 [25] | 27 ± 9 | 25 ± 6 | 73 ± 12 | 74 ± 13 | 114 ± 17 | 107 ± 10 | NA | NA |
II: 46 (96) IV 2 (4) |
II: 19 (91) IV: 2 (9) |
5678 ± 4847 | 5419 ± 5331 |
| Zhang et al. 2015 [24] | 30.6 ± 6 | 30.9 ± 6.3 | 115 ± 53 | 117 ± 56 | 133 ± 49 | 132 ± 47 | 86 ± 24 | 185 ± 24 |
I: II 0 0 III: IV 21 |
I–II: 0 0 III–IV: 21 |
2895.7 ± 1497.5 | 2910.5 ± 1490.4 |
| Shah et al. 2014 [23] | 22.5 ± 4.1 | 22.6 ± 3.4 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Altenberger et al. 2014 [27] | 24 ± 5 | 24 ± 5 | 73 ± 12 | 73 ± 12 | 120 ± 14 | 120 ± 18 | NA | NA |
IIIb: 61 (96.8) IV: 2 (3.2) |
IIIb: 53 (92.9) IV: 4 (7.1) |
3230.7 ± 2955.1 | 3593 ± 1172.3 |
| Llorens et al. 2012 [19] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 5482 ± 4711 | 5988 ± 6027 |
| Kurt et al. 2010 [17] | 25.4 ± 4.3 | 25.4 ± 4.5 | 93.8 ± 23 | 96.6 ± 23.7 | 128.3 ± 20.6 | 116.3 ± 21.3 | 71.1 ± 10.7 | 78.8 ± 20.2 |
III: 21 (72.4) IV: 8 (27.6) |
III: 24 (80) IV: 6 (20) |
NA | NA |
| Nieminen et al. 2008 [21] |
LS-1: 25 ± 4.9 LS-2: 25 ± 5.3 |
25 ± 4.9 |
LS-1: 75 ± 13 LS-2: 74 ± 12 |
78 ± 13 |
LS-1: 123 ± 18 LS-2: 124 ± 17 |
124 ± 15 | NA | NA |
LS-1: IIIB: 4 (82) IV: 18 (18) LS-2: IIIB: 6 (93) IV: 7 (7) |
IIIB: 87 (85) IV: 15 (15) |
LS-1: 7557 ± 6680 LS-2: 5414.3 ± 4961.7 |
5809.3 ± 6489 |
| Mavrogeni et al. 2007 [20] | 22 ± 6 | 22 ± 5 | 78 ± 13 | 80 ± 13 | NA | NA | NA | NA | III or IV: 25 (100) | III or IV: 25 (100) | NA | NA |
| Parissis et al. 2007 [22] | 23 ± 6 | 22 ± 5 | 83 ± 15 | 86 ± 17 | 99 ± 11 | 100 ± 12 | NA | NA | 3.3 ± 0.7 | 3.4 ± 0.6 | NA | NA |
| Lilleberg et al. 2007 [18] | 25 ± 5 | 28 ± 6 | 63 ± 9 | 66 ± 10 | 116 ± 11 | 125 ± 17 | 71 ± 8 | 74 ± 9 | NA | NA | 8524.7 ± 8862.9 | 5353.3 ± 4702.1 |
| Parissis et al. 2006 [11] | 22 ± 4 | 23 ± 4 | 74 ± 9 | 73 ± 8 | 117 ± 14 | 110 ± 15 | 73 ± 8 | 71 ± 8 |
III: 7 (41.1) IV: 10 (58.9) |
III: 4 (50%) IV: 4 (50%) |
1547 ± 347 | 1302 ± 302 |
| Parissis et al. 2005 [13] | 20 ± 5 | 23 ± 6 | NA | NA | 103 ± 13 | 109 ± 13 | 65 ± 8 | 68 ± 6 | 3.7 ± 0.5 | 3.5 ± 0.5 | NA | NA |
Data are reported as n (%) or mean ± SD
BMI body mass index, DBP diastolic blood pressure, LS-1 1-mg capsule of levosimendan once daily group, LS-2 1-mg capsule of levosimendan twice daily group, LVEF left ventricular ejection fraction, NA not available, NT-proBNP N-terminal-pro-B-type natriuretic peptide, NYHA New York Heart Association, SBP systolic blood pressure
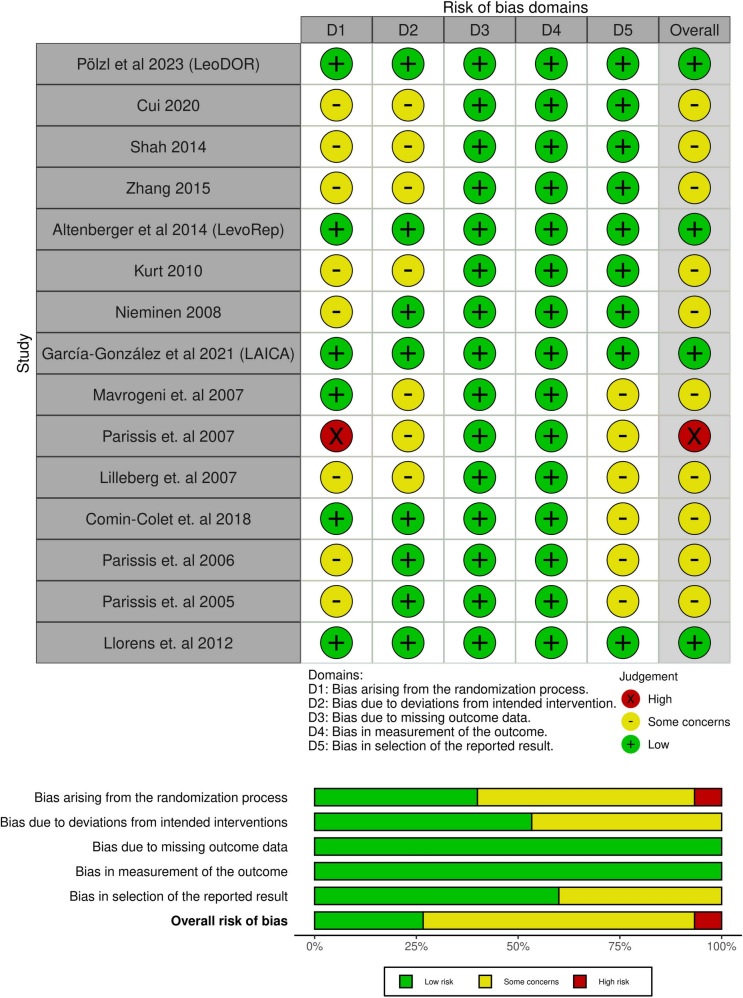
Risk of Bias and Certainty of Evidence
ROB-II revealed that only one study exhibited a high risk of bias, while four studies exhibited a low risk of bias. Conversely, the others raised some concerns of bias across various domains (Fig. 2). The evidence certainty level is elaborated in a GRADE framework profile in Table 3. Across the included trials, there was a very low level of certainty for all pooled outcomes, except for all-cause mortality, which was assessed to have a low certainty level.
Fig. 2.
Quality assessment of risk of bias in the included trials. The upper panel presents a schematic representation of risks (low = green, unclear = yellow, and high = red) for specific types of biases of each study in the review. The lower panel presents risks (low = green, unclear = yellow, and high = red) for the subtypes of biases of the combination of studies included in this review
Table 3.
GRADE evidence profile
| Certainty assessment | ||||||
|---|---|---|---|---|---|---|
| Participants (studies) Follow-up |
Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence |
| LVEF change | ||||||
| 323 (7 RCTs) | Seriousa | Very seriousb | Not serious | Very seriousc | None |
⨁◯◯◯ Very low |
| All-cause mortality | ||||||
| 636 (7 RCTs) | Seriousa | Not serious | Not serious | Seriousd | None |
⨁⨁◯◯ Low |
| BNP change | ||||||
| 157 (3 RCTs) | Seriousa | Not serious | Not serious | Very seriousc | None |
⨁◯◯◯ Very low |
| Event-free survival | ||||||
| 465 (5 RCTs) | Seriousa | Seriouse | Not serious | Very seriousd | None |
⨁◯◯◯ Very low |
| All-cause re-hospitalization | ||||||
| 260 (4 RCTs) | Seriousa | Seriouse | Not serious | Very seriousd | None |
⨁◯◯◯ Very low |
| Any adverse event | ||||||
| 462 (7 RCTs) | Seriousa | Not serious | Not serious | Very seriousd | None |
⨁◯◯◯ Very low |
| Hypotension | ||||||
| 327 (5 RCTs) | Seriousa | Not serious | Not serious | Very seriousd | None |
⨁◯◯◯ Very low |
| Tachycardia | ||||||
| 463 (4 RCTs) | Seriousa | Not serious | Not serious | Very seriousd | None |
⨁◯◯◯ Very low |
BNP brain natriuretic peptide, CI confidence interval, GRADE Grading of Recommendations Assessment, Development, and Evaluation, LVEF left ventricular ejection fraction, RCT randomized controlled trial
aAll the included trials showed an overall some concerns of bias
bI-squared > 75%
cA wide CI that does not exclude the appreciable harm/benefit, with total participants less than 400
dA wide CI that does not exclude the appreciable harm/benefit, with low number of events
eI-squared > 50%
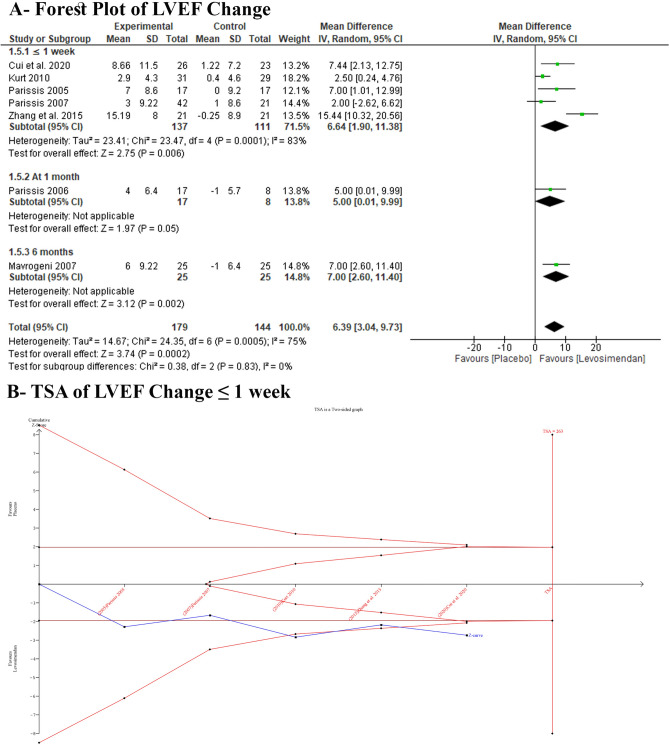
Primary Outcomes: LVEF
Levosimendan demonstrated a statistically significant enhancement in LVEF compared with placebo (MD 6.39 [95% CI 3.04–9.73], I2 = 75%) (Fig. 3A). Excluding Zhang et al. 2015 from the subgroup analysis for the ≤ 1-week revealed a consistent statistically significant difference between the study groups (MD 4.09 [95% CI 1.41–6.67], I2 = 27%) (Table S3, see the electronic supplementary material). A TSA for LVEF change at ≤ 1 week declared that the cumulative z-curve surpassed the traditional and TSA boundaries, establishing sufficient and conclusive evidence (Fig. 3B).
Fig. 3.
Forest plot and TSA of LVEF change: A forest plot; B TSA LVEF change ≤ 1 week. CI confidence interval, LVEF left ventricular ejection fraction, TSA trial sequential analysis
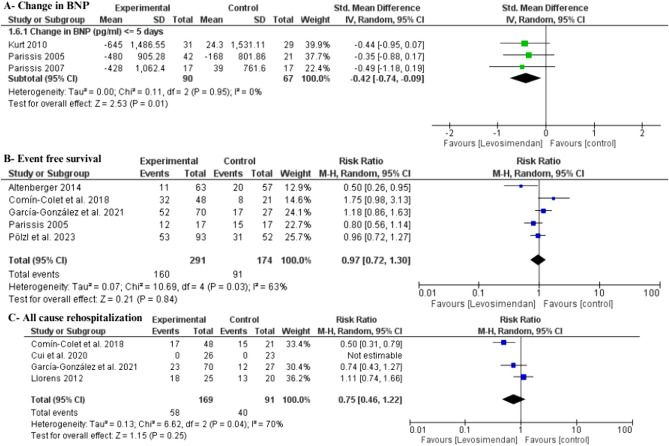
Secondary Efficacy Outcomes
Levosimendan was associated with a significant decrease in all-cause mortality compared with placebo (RR 0.60 [95% CI 0.40–90], I2 = 9%) (Fig. 4). Additionally, levosimendan demonstrated a significant reduction in BNP level (SMD −0.42 [95% CI −0.74 to −0.09], I2 = 0%) without any heterogeneity (Fig. 5A). A TSA for BNP change at ≤ 1 week declared that the z-curve surpassed the traditional and TSA boundaries, establishing sufficient and conclusive evidence (Figure S1, see the electronic supplementary material).
Fig. 4.
Forest plot of all-cause mortality. CI confidence interval
Fig. 5.
Forest plot of secondary efficacy outcomes: A change in BNP; B event-free survival; C all-cause rehospitalization. BNP brain natriuretic peptide, CI confidence interval, Std. standardized
However, no significant difference was observed between levosimendan and placebo in terms of event-free survival (RR 0.97 [95% CI 0.72–1.30], I2 = 63%) (Fig. 5B). Subgroup analysis was also done according to the time point; all subgroups had no significant differences (Figure S2). Heterogeneity in the 5-month subgroup was resolved by excluding the study Parissis et al. (2005) (I2 = 32%, P = 0.23), and the results remained insignificant (RR 1.34 [95% CI 0.92–1.95]) (Table S4). Furthermore, no distinction was found between levosimendan and placebo concerning all-cause rehospitalization (RR 0.75 [95% CI 0.46–1.22], I2 = 70%) (Fig. 5C). Subgroup analysis was consistent according to the time points, with no significant difference in all subgroups (Figure S3). Heterogeneity in the 6-month subgroup was resolved by excluding the study Llorens et al. (2012) (I2 = 0%), and the results became statistically significant (RR 0.54 [95% CI 0.37–0.79]) (Table S5).
Safety Outcomes
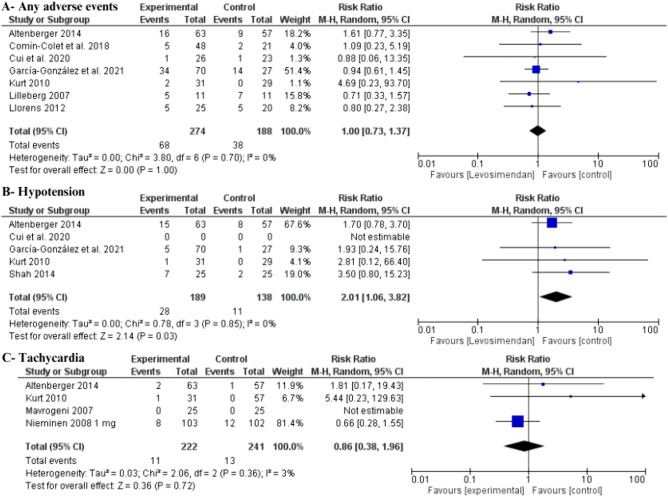
While levosimendan did not show an overall elevated risk of any adverse events (RR 1 [95% CI 0.73–1.37], I2 = 0%) (Fig. 6A), it was associated with a statistically significant increase in the risk of hypotension (RR 2.01 [95% CI 1.06–3.82], I2 = 0%) (Fig. 6B). There was no statistically significant difference in tachycardia events between groups (RR 0.86 [95% CI 0.38–1.96], I2 = 3%) (Fig. 6C). Additionally, the requirement for renal replacement therapy/dialysis was investigated by Shah et al. 2014, with an event rate of 16% observed in both groups.
Fig. 6.
Forest plot of safety outcomes: A any adverse events; B hypotension; C tachycardia. CI confidence interval
Discussion
The key findings are summarized as follows: (1) intermittent levosimendan is associated with a statistically significant increase in LVEF; (2) levosimendan is associated with a statistically significant reduction in BNP; (3) levosimendan is associated with a statistically significant reduction in all-cause mortality; (4) levosimendan is not associated with a reduction in all-cause rehospitalization; (5) levosimendan is not associated with increased adverse events apart from hypotension.
Ambulatory use of intermittent levosimendan in advanced heart failure is under investigation. Advanced heart failure patients often tolerate GDMT with disease-modifying drugs poorly. Tomasoni et al. demonstrated that a minority of advanced heart failure patients in the HELP-HF registry were able to tolerate target doses of GDMT [38]. The American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Failure Society of America (HFSA) recommend against ambulatory use of IV inotropic agents such as dobutamine and milrinone, except in palliative care or bridging to advanced therapy settings due to lack of survival benefit [14, 39, 40]. Novel agents such as the myosin activator omecamtiv mecarbil were developed as adjunct therapies to reduce severe heart failure patient risk. However, the Food and Drug Administration (FDA) declined approval due to lack of survival benefit [41]. A great unmet need exists for a therapeutic option to prevent disease progression, decrease hospitalizations, lower mortality, and enhance the quality of life in advanced heart failure patients. Given its distinct mechanism of action as an inodilator calcium-sensitizing agent with cardioprotective properties, intermittent ambulatory levosimendan holds promise for enhancing cardiovascular outcomes and improving quality of life in advanced heart failure patients.
Despite mixed evidence in several relatively small RCTs, our findings suggest that intermittent levosimendan may improve cardiovascular outcomes in heart failure patients, primarily by reducing all-cause mortality. These positive clinical outcomes might stem from improving hemodynamic and neurohormonal profiles, as manifested by increased LVEF and reduced natriuretic peptides in our results. Our results align with a study including eight RCTs and seven observational studies, which showed decreased cardiovascular mortality, improved New York Heart Association (NYHA) class, and health-related quality of life with ambulatory levosimendan use; however, that study was limited by heterogeneity and the small size of included studies [42]. In keeping with our findings, Cui et al. (2021) also found that levosimendan reduced BNP levels and increased LVEF in patients with advanced heart failure [43]. Moreover, the RELEVANT-HF registry, designed to acquire real-world data for patients with advanced heart failure receiving levosimendan, also demonstrated that patients experienced a shorter length of hospital stay in the 6 months following the initiation of treatment compared with the 6 months prior to treatment [43].
Levosimendan may also exert a positive impact on the pulmonary circulation and the right ventricle, which can contribute to improved cardiovascular outcomes. A systematic review and meta-analysis involving 390 patients showed a significant increase in right ventricular (RV) fractional area change, tricuspid annular plane systolic excursion, and tricuspid annular peak systolic velocity and a significant decrease in pulmonary pressures with levosimendan use [44]. These findings are of particular importance in the advanced heart failure population that may seek durable left ventricular assist device (LVAD) options, as up to 24% develop right heart failure following LVAD implantation, leading to worse cardiovascular outcomes [45].
Furthermore, there is evidence that levosimendan improves long-term kidney function in patients with advanced heart failure. Studies showed increased glomerular filtration rate and decreased creatinine with levosimendan use [46–48]. This effect may be explained by favorable cardiac effects or potential extra-cardiac mechanisms. That said, Shah et al. failed to demonstrate a reduction in the need for renal replacement therapies with the use of levosimendan.
Our results suggest that levosimendan is well tolerated in patients with advanced heart failure with no increased risk of adverse events apart from an increase in hypotensive events. However, increased hypotension with levosimendan did not correspond to a rise in all-cause mortality. Despite its inotropic properties, levosimendan did not exhibit a significant increase in tachycardic events in our results. This finding can be explained within the context that levosimendan does not increase intracellular calcium or oxygen consumption, leading to less likely ventricular arrhythmias during treatment [49]. We may demonstrate that intermittent ambulatory levosimendan use leads to improvements in LVEF, BNP, and all-cause mortality without an increased hazard of overall adverse events.
However, this study cannot demonstrate the risk–benefit ratio of intermittent levosimendan for patients with advanced heart failure as there was not enough data to pool and discuss the whole safety profile of the treatment (Table S6, see the electronic supplementary material).
Limitations
This study resolved some of the limitations of Cui et al. (2021) as we unified the comparator arm.
However, this review should be interpreted considering several limitations. (1) There were heterogeneous baseline patient characteristics. (2) There was predominantly male representation (76% of included patients). (3) There was variation in dosing regimens across different RCTs; some studies used a bolus dose while others did not, and different studies used different maintenance dosing. (4) Although all other studies used IV levosimendan, in the study by Nieminen et al., patients received oral levosimendan. (5) There was noted heterogeneity among some outcomes, including change in LVEF, event-free survival, and all-cause rehospitalization. (6) There was limited investigation into the effect of levosimendan on patient-reported quality of life in most studies.
Furthermore, this meta-analysis identified some inconsistencies in its findings. While levosimendan was associated with significant improvements in LVEF, BNP levels, and overall mortality, it did not show a significant impact on all-cause rehospitalization rates, event-free survival rates, or adverse events. A subgroup analysis based on the duration of follow-up for the outcomes of all-cause rehospitalization rate and event-free survival rate also revealed no significant differences between the study groups across all subgroups (Figures S2 and S3, see the electronic supplementary material). The inconsistency in these outcomes suggests that the benefits of levosimendan may be limited or context specific. However, this discrepancy in these outcomes may be attributed to the variability of the RCTs included in each outcome analysis and the absence of data related to specific follow-up time points in some studies.
Implications for Future Research
Future adequately powered trials are needed to draw definitive conclusions regarding the efficacy of ambulatory levosimendan on cardiovascular outcomes, including cardiovascular hospitalizations, cardiovascular mortality, and quality-of-life measures. Additionally, the optimal dosing regimen needs to be refined. Subsequent studies should investigate whether the effects of levosimendan are consistent across the spectrum of heart failure ejection fraction subtypes, heart failure etiologies, and different follow-up periods. Exploring levosimendan's efficacy in patients with RV heart failure is also an important area for exploration.
Conclusion
Our systematic review and meta-analysis suggest that intermittent levosimendan is associated with a significantly higher LVEF, lower BNP levels, and lower all-cause mortality in patients with chronic heart failure. However, levosimendan use was not associated with a reduction in all-cause rehospitalization.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Declarations
Author contributions
M.A., B.P. and C.B. conceived the idea. B.A., A.S.E. and M.A. designed the research workflow. B.A. and M.A. searched the databases. A.M.A., M.E., S.E. and A.R.S. screened the retrieved records, extracted relevant data, assessed the quality of evidence, and B.A. and A.S.E resolved the conflicts. Y.S. performed the analysis. R.G., Y.S. and A.S.E. wrote the final manuscript. B.A., B.P., and C.B. supervised the project. All authors have read and agreed to the final version of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Funding
We received no funding for this study.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Footnotes
Ahmed Saad Elsaeidy and Mohamed Abuelazm have equal contributions and are co-first authors.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. [DOI] [PMC free article] [PubMed]
- 2.Sterling MR, Ringel JB, Pinheiro LC, Safford MM, Levitan EB, Phillips E, et al. Social determinants of health and 90-day mortality after hospitalization for heart failure in the regards study. J Am Heart Assoc. 2020;9. [DOI] [PMC free article] [PubMed]
- 3.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254-743. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Cogswell ME, Dana Flanders W, Hong Y, Zhang Z, Loustalot F, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among us adults. JAMA. 2012;307:1273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The efficacy and safety of new potassium binders on renin–angiotensin–aldosterone system inhibitor optimization in heart failure patients: a systematic review and meta‐analysis—Abuelazm—2024—ESC Heart Failure—Wiley Online Library [Internet]. 10.1002/ehf2.14588. Cited 19 Mar 2024. [DOI] [PMC free article] [PubMed]
- 6.Repetitive LevosimenDan infusions fOR patients with advanced chronic heart failure in the vulnerable post-discharge period: the multinational randomized LeoDOR trial. Eur Heart J Oxford Academic [Internet]. https://academic.oup.com/eurheartj/article/44/Supplement_2/ehad655.798/7391695. Cited 9 Dec 2023. [DOI] [PubMed]
- 7.Grossini E, Farruggio S, Pierelli D, Bolzani V, Rossi L, Pollesello P, et al. Levosimendan improves oxidative balance in cardiogenic shock/low cardiac output patients. J Clin Med. 2020;9:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchez S, Fedele F, Giannakoulas G, Gustafsson F, Harjola VP, Karason K, et al. Levosimendan in acute and advanced heart failure: an expert perspective on posology and therapeutic application. Cardiovasc Drugs Ther. 2018;32:617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis GS, Bartos JA, Adatya S. Inotropes. J Am Coll Cardiol. 2014;63:2069–78. [DOI] [PubMed] [Google Scholar]
- 10.Nieminen MS, Akkila J, Hasenfuss G, Kleber FX, Lehtonen LA, Mitrovic V, et al. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1903–12. [DOI] [PubMed] [Google Scholar]
- 11.Parissis JT, Adamopoulos S, Farmakis D, Filippatos G, Paraskevaidis I, Panou F, et al. Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure. Heart. 2006;92:1768–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Packer M, Colucci W, Fisher L, Massie BM, Teerlink JR, Young J, et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 2013;1:103–11. [DOI] [PubMed] [Google Scholar]
- 13.Parissis JT, Panou F, Farmakis D, Adamopoulos S, Filippatos G, Paraskevaidis I, et al. Effects of Levosimendan on markers of left ventricular diastolic function and neurohormonal activation in patients with advanced heart failure. Am J Cardiol. 2005;96:423–6. [DOI] [PubMed] [Google Scholar]
- 14.Thackray S, Easthaugh J, Freemantle N, Cleland JGF. The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure—a meta-regression analysis. Eur J Heart Fail. 2002;4:515–29. [DOI] [PubMed] [Google Scholar]
- 15.Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. Cardiology. JAMA. JAMA Network [Internet]. https://jamanetwork.com/journals/jama/fullarticle/194768. Cited 28 Mar 2024. [DOI] [PubMed]
- 16.Efficacy and safety of intermittent repeated levosimendan infusions in advanced heart failure patients: the LAICA study—García‐González—2021—ESC heart failure—Wiley Online Library [Internet]. 10.1002/ehf2.13670. Cited 9 Dec 2023. [DOI] [PMC free article] [PubMed]
- 17.Kurt IH, Yavuzer K, Batur MK. Short-term effect of levosimendan on free light chain kappa and lambda levels in patients with decompensated chronic heart failure. Heart Vessels. 2010;25:392–9. [DOI] [PubMed] [Google Scholar]
- 18.Lilleberg J, Laine M, Palkama T, Kivikko M, Pohjanjousi P, Kupari M. Duration of the haemodynamic action of a 24-h infusion of levosimendan in patients with congestive heart failure. Eur J Heart Fail. 2007;9:75–82. [DOI] [PubMed] [Google Scholar]
- 19.Llorens P, Miro O, Roman F, Zapater P, Carbajosa-Dalmau J, Llanos L. Efficacy of early administration of levosimendan in emergency department in patients with acute heart failure: a randomized pilot clinical trial. Emergencias. 2012;24:268–76. [Google Scholar]
- 20.Mavrogeni S, Giamouzis G, Papadopoulou E, Thomopoulou S, Dritsas A, Athanasopoulos G, et al. A 6-month follow-up of intermittent levosimendan administration effect on systolic function, specific activity questionnaire, and arrhythmia in advanced heart failure. J Card Fail. 2007;13:556–9. [DOI] [PubMed] [Google Scholar]
- 21.Nieminen MS, Cleland JGF, Eha J, Belenkov Y, Kivikko M, Põder P, et al. Oral levosimendan in patients with severe chronic heart failure—the PERSIST study. Eur J Heart Fail. 2008;10:1246–54. [DOI] [PubMed] [Google Scholar]
- 22.Effects of levosimendan on quality of life and emotional stress in advanced heart failure patients. Cardiovasc Drugs Ther. [Internet]. 10.1007/s10557-007-6034-2. Cited 9 Dec 2023. [DOI] [PubMed]
- 23.Shah B, Sharma P, Brahmbhatt A, Shah R, Rathod B, Shastri N, et al. Study of levosimendan during off-pump coronary artery bypass grafting in patients with LV dysfunction: a double-blind randomized study. Indian J Pharmacol. 2014;46:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang D, Yao Y, Qian J, Huang J. Levosimendan improves clinical outcomes of refractory heart failure in elderly chinese patients. Med Sci Monit. 2015;21:2439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comín-Colet J, Manito N, Segovia-Cubero J, Delgado J, García Pinilla JM, Almenar L, et al. Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentre randomised trial. Eur J Heart Fail. 2018;20:1128–36. [DOI] [PubMed] [Google Scholar]
- 26.Cui XR, Yang XH, Li RB, Wang D, Jia M, Bai L, et al. Short-term efficacy and safety of levosimendan in patients with chronic systolic heart failure. Cardiovasc J Afr. 2020;31:196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altenberger J, Parissis JT, Costard-Jaeckle A, Winter A, Ebner C, Karavidas A, et al. Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial. Eur J Heart Fail. 2014;16:898–906. [DOI] [PubMed] [Google Scholar]
- 28.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ [Internet]. https://www.bmj.com/content/372/bmj.n71. Cited 9 Dec 2023.
- 29.Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions—PubMed [Internet]. https://pubmed.ncbi.nlm.nih.gov/31643080/. Cited 9 Dec 2023. [DOI] [PMC free article] [PubMed]
- 30.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898. [DOI] [PubMed] [Google Scholar]
- 31.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collaboration C. Review Manager (RevMan)[Computer program]. Version 5.3. 5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. The; 2014.
- 34.Abuelazm M, Ali S, AlBarakat MM, Mahmoud A, Tanashat M, Suilik HA, et al. Istaroxime for patients with acute heart failure: a systematic review and meta-analysis of randomized controlled trials. Diseases. 2023;11:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions: cochrane book series.
- 36.Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elsaeidy AS, Taha AM, Abuelazm M, Soliman Y, Ali MA, Alassiri AK, et al. Efficacy and safety of extracorporeal membrane oxygenation for cardiogenic shock complicating myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2024;24:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomasoni D, Pagnesi M, Colombo G, Chiarito M, Stolfo D, Baldetti L, et al. Guideline-directed medical therapy in severe heart failure with reduced ejection fraction: an analysis from the HELP-HF registry. Eur J Heart Fail. 2024;26:327–37. [DOI] [PubMed] [Google Scholar]
- 39.Ambulatory inotrope infusions in advanced heart failure: a systematic review and meta-analysis—PMC [Internet]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6119101/. Cited 8 Mar 2024. [DOI] [PMC free article] [PubMed]
- 40.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-1032. [DOI] [PubMed] [Google Scholar]
- 41.Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med [Internet]. 10.1056/NEJMoa2025797. Cited 2 Aug 2024. [DOI] [PubMed]
- 42.Elsherbini H, Soliman O, Zijderhand C, Lenzen M, Hoeks SE, Kaddoura R, et al. Intermittent levosimendan infusion in ambulatory patients with end-stage heart failure: a systematic review and meta-analysis of 984 patients. Heart Fail Rev. 2022;27:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui D, Liao Y, Li G, Chen Y. Levosimendan can improve the level of B-type natriuretic peptide and the left ventricular ejection fraction of patients with advanced heart failure: a meta-analysis of randomized controlled trials. Am J Cardiovasc Drugs. 2021;21:73–81. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, Wei Z, Zhang C, Lu C, Zeng Z. The effect of levosimendan on right ventricular function in patients with heart dysfunction: a systematic review and meta-analysis. Sci Rep. 2021;11:24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kapelios CJ, Lund LH, Wever-Pinzon O, Selzman CH, Myers SL, Cantor RS, et al. Right heart failure following left ventricular device implantation: natural history, risk factors, and outcomes: an analysis of the STS INTERMACS database. Circ Heart Fail. 2022;15: e008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lannemyr L, Ricksten S-E, Rundqvist B, Andersson B, Bartfay S-E, Ljungman C, et al. Differential effects of levosimendan and dobutamine on glomerular filtration rate in patients with heart failure and renal impairment:a randomized double-blind controlled trial. J Am Heart Assoc. 2018;7: e008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yilmaz MB, Yalta K, Yontar C, Karadas F, Erdem A, Turgut OO, et al. Levosimendan improves renal function in patients with acute decompensated heart failure: comparison with dobutamine. Cardiovasc Drugs Ther. 2007;21:431–5. [DOI] [PubMed] [Google Scholar]
- 48.Zemljic G, Bunc M, Yazdanbakhsh AP, Vrtovec B. Levosimendan improves renal function in patients with advanced chronic heart failure awaiting cardiac transplantation. J Card Fail. 2007;13:417–21. [DOI] [PubMed] [Google Scholar]
- 49.Moreno N, Tavares-Silva M, Lourenço AP, Oliveira-Pinto J, Henriques-Coelho T, Leite-Moreira AF. Levosimendan: The current situation and new prospects. Rev Port Cardiol Orgao Of Soc Port Cardiol Port J Cardiol Off J Port Soc Cardiol. 2014;33:795–800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.