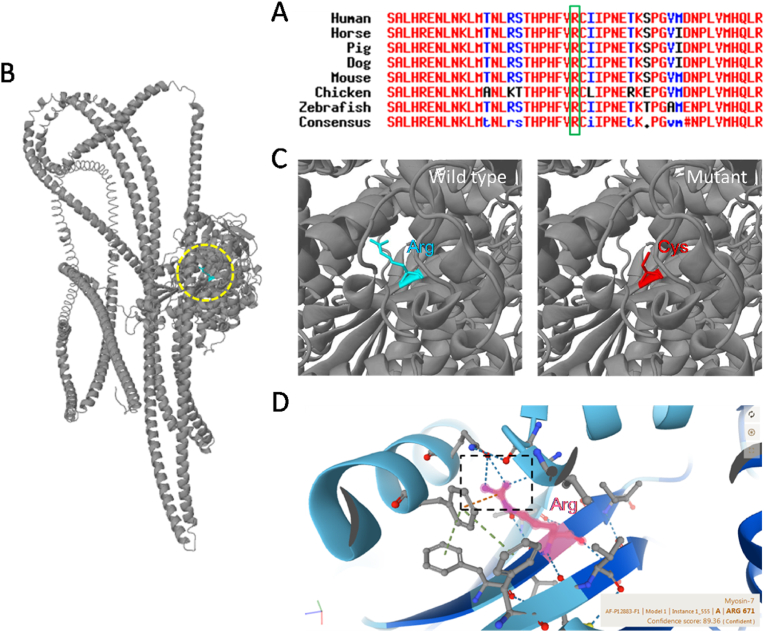
Fig. 3.
Evolutionary conservation analysis and structural bioinformatics analysis. A. Alignment of the amino acid sequences of Myosin-7 indicated that the arginine residue replaced owing to mutation is evolutionarily conserved across species. The green square marks the arginine residue. B, C. Structural bioinformatics analysis with Missense3D. The spatial position of the mutation site in the motor/head domain of Myosin-7 protein was circled by yellow dashed line in (B). The analysis results indicated that the substitution leads to the loss of cation-Pi interaction with Phe 489 and hydrogen bonds with neighboring residues (WT: Arg671-NH2 … OG1-Thr177, Arg671-NH1 … OE1-Gln486, Arg671-NH2 … OE1-Gln486, Arg671-NH2 … OD1-Asn486 vs. Mutant: Asn696-ND2 … SG-Cys671). D. The wild type 3D structure from AlphaFold Protein Structure Database to show the interaction of Arg671 with neighboring amino acids. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)