Abstract
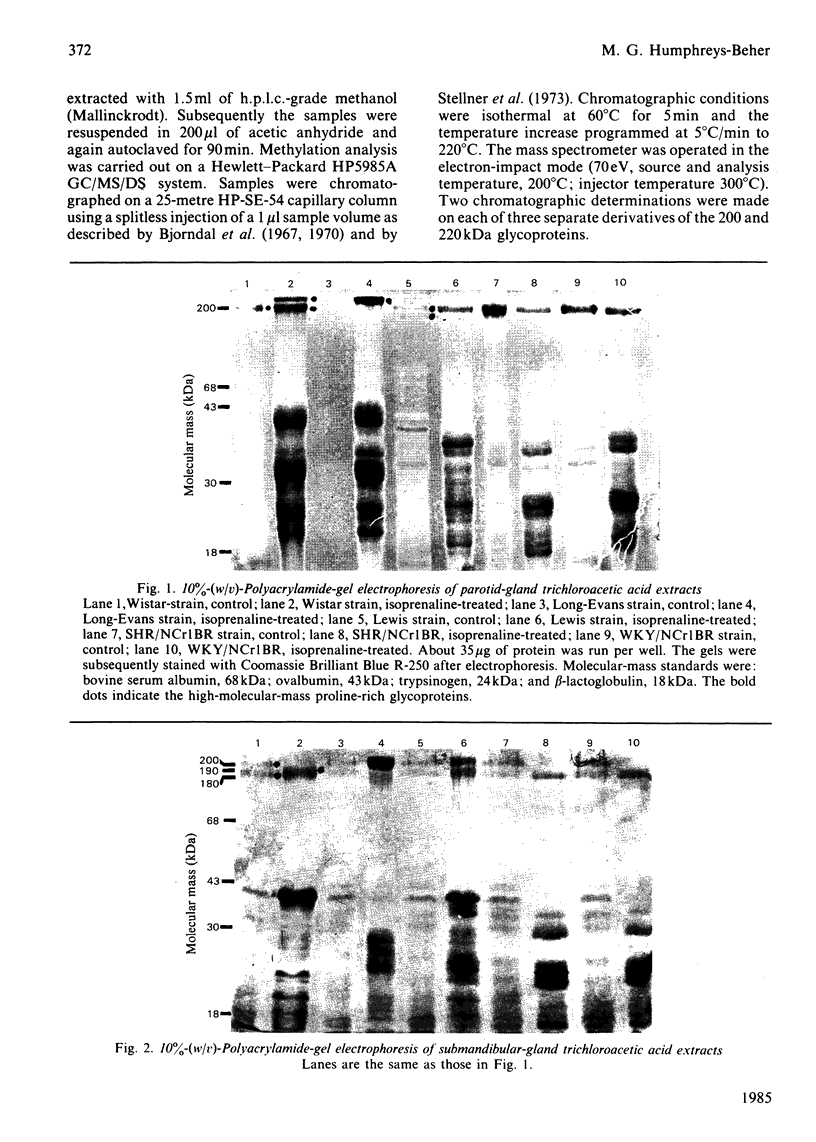
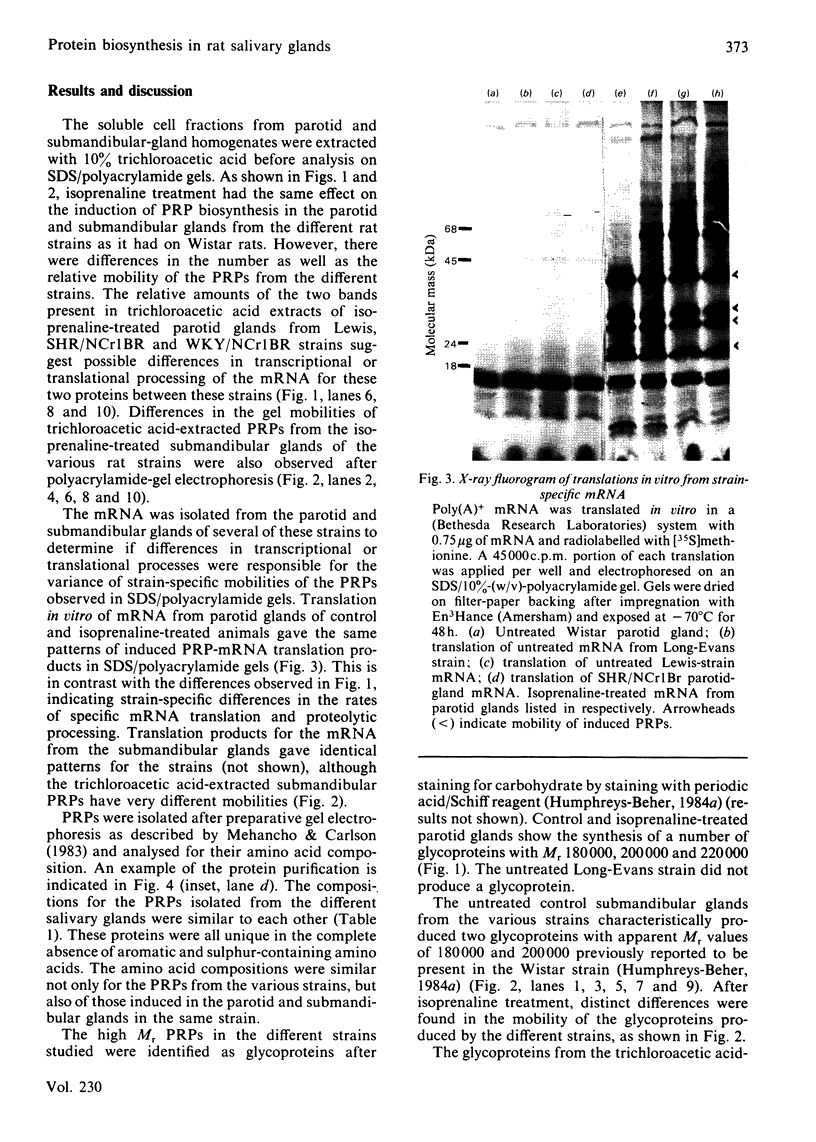
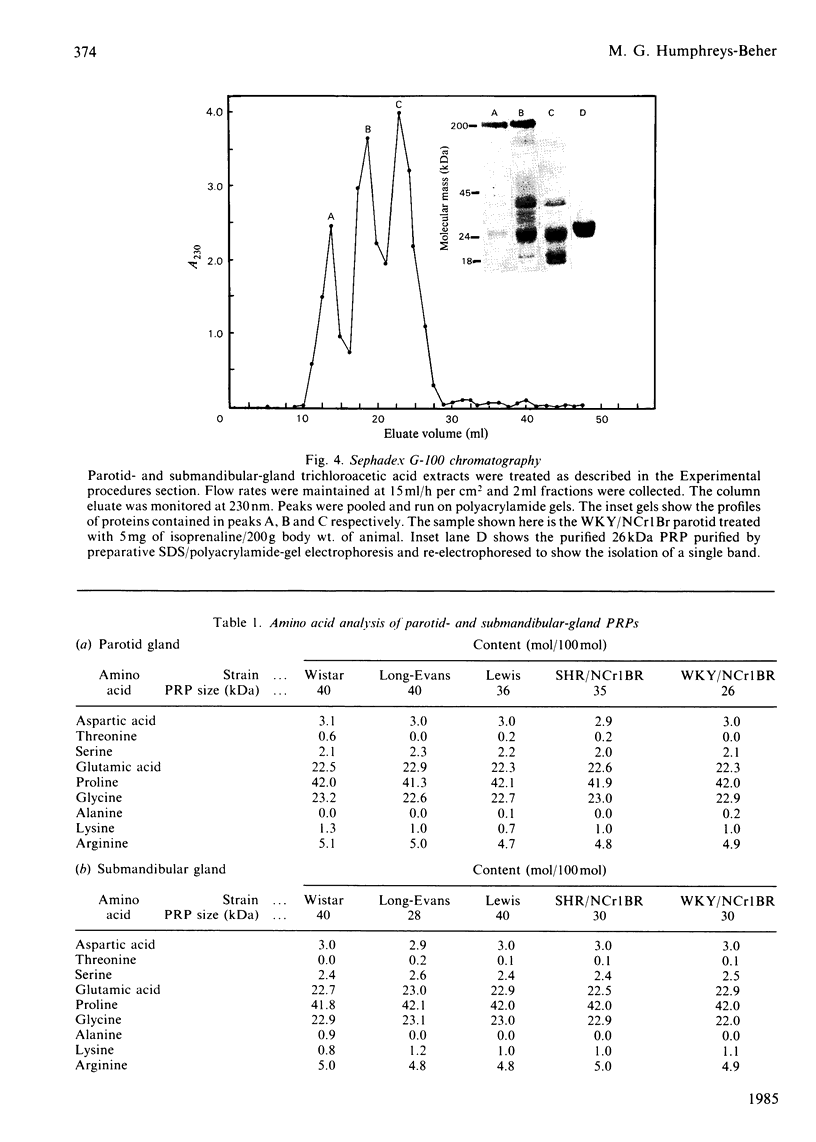
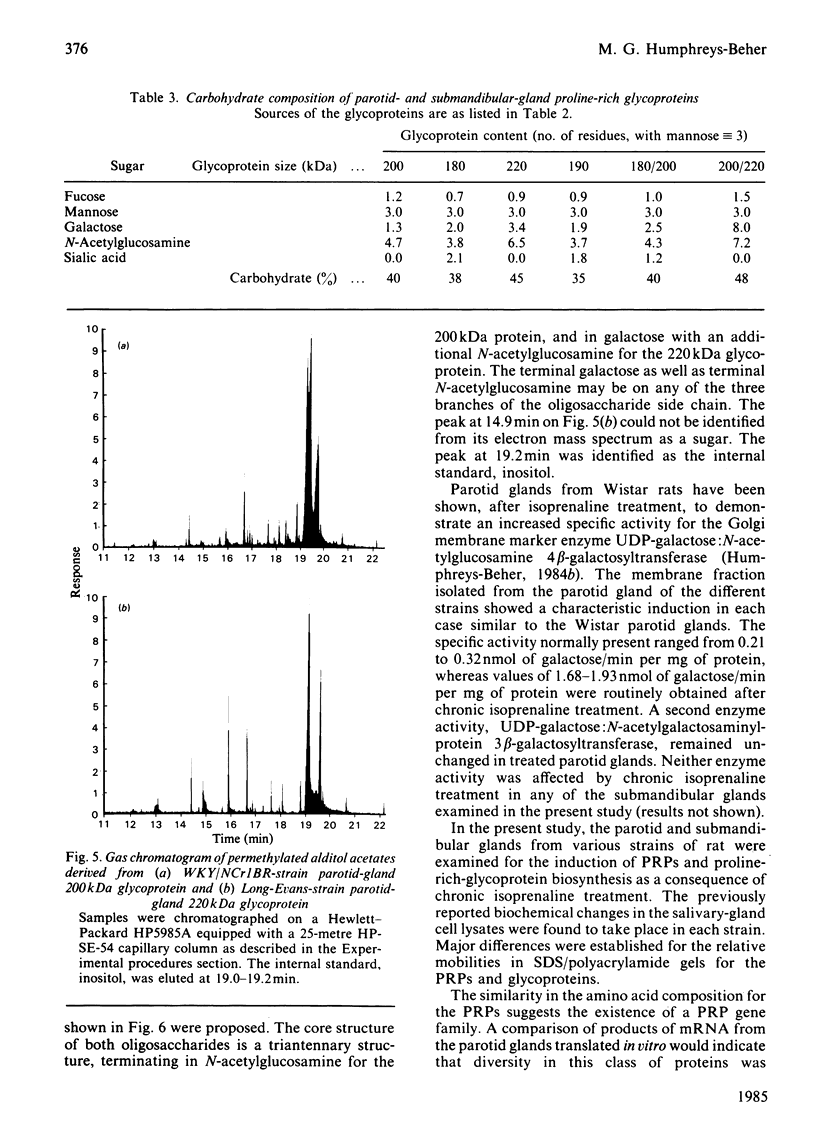
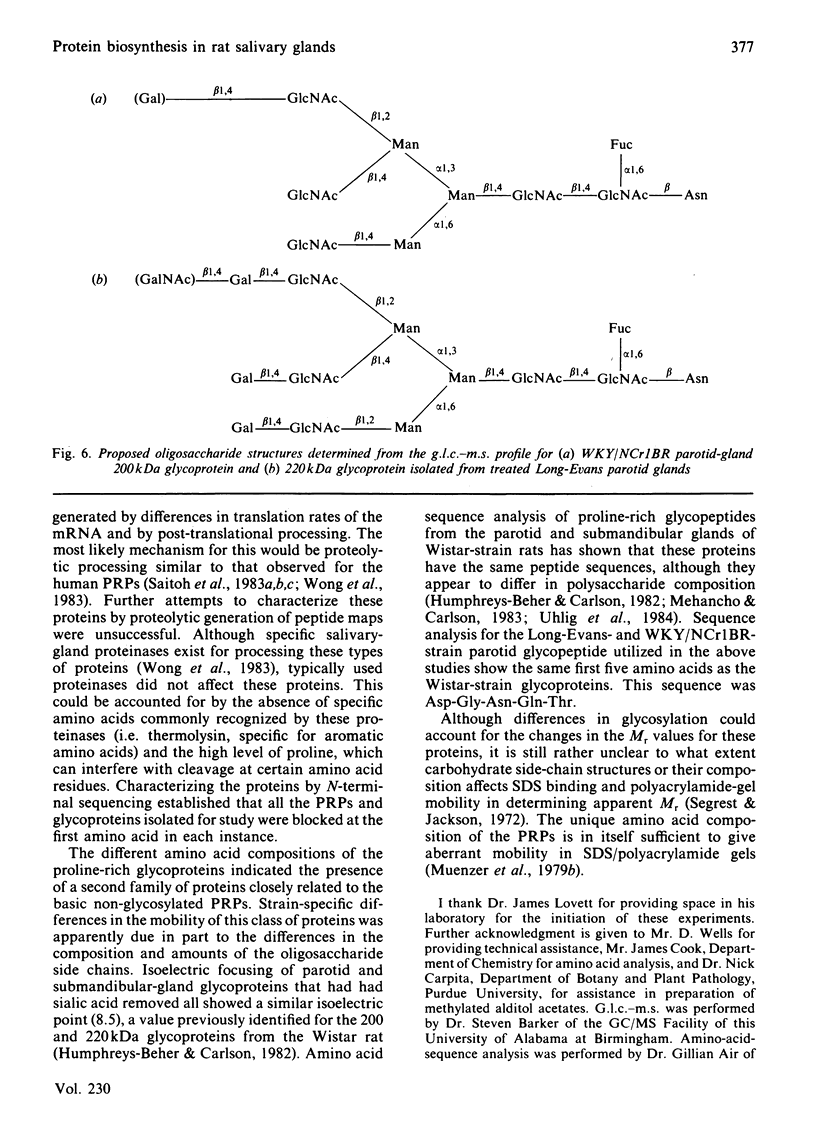
Parotid and submandibular glands were isolated from five strains of rat after chronic injection of the beta-adrenergic receptor agonist isoprenaline (isoproterenol). The glands were observed to have undergone a marked increase in wet weight, owing to hypertrophy and hyperplasia. The 100 000 g soluble fraction of gland cell lysates were extracted with 10% (w/v) trichloroacetic acid, and the soluble material subsequently analysed by SDS (sodium dodecyl sulphate)/polyacrylamide-gel electrophoresis. By this procedure, evidence was obtained for the induction, in isoprenaline-treated parotid and submandibular glands, of proline-rich proteins with apparent Mr values ranging from 20 000 to 40 000. Heterogeneity was evident in the proteins produced for a specific gland between the rat strains, although the amino acid compositions were the same. Products from induced mRNAs translated in vitro had similar mobilities in SDS/polyacrylamide gels, despite the apparent difference in mobility of trichloracetic acid-extracted proline-rich proteins from the various strains. Strain-specific differences were noted for the proline-rich glycoproteins from control salivary glands as well as those induced as a consequence of isoprenaline treatment. Although the glycoproteins had similar amino acid compositions, there was considerable heterogeneity in the carbohydrate compositions for these proteins, suggesting that the differences were the result of post-translational modifications during glycosylation. Induction of the increased activity of the Golgi membrane marker enzyme UDP-galactose:2-acetamido-2-deoxy-D-glucosamine 4 beta-galactosyltransferase (EC 2.4.1.22) occurred to the same extent in the parotid glands of all strains examined. There was no change in the specific activity of a second enzyme, UDP-galactose:N-acetylgalactosaminyl-protein 3 beta-galactosyltransferase (no EC designation).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A., Connell G. E. Purification and partial characterization of four proteins from human parotid saliva. Biochem J. 1971 Jul;123(3):455–464. doi: 10.1042/bj1230455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson D. M., David J., Rutter W. J. Galactosyltransferase activities in pancreas, liver and gut of the developing rat. Arch Biochem Biophys. 1973 Aug;157(2):605–612. doi: 10.1016/0003-9861(73)90680-2. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Humphreys-Beher M. G., Hollis D. L., Carlson D. M. Comparative developmental analysis of the parotid, submandibular and sublingual glands in the neonatal rat. Biochem J. 1982 Jun 15;204(3):673–679. doi: 10.1042/bj2040673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys-Beher M. G. Isolation and characterization of UDP-galactose:N-acetylglucosamine 4 beta-galactosyltransferase activity induced in rat parotid glands treated with isoproterenol. J Biol Chem. 1984 May 10;259(9):5797–5802. [PubMed] [Google Scholar]
- Humphreys-Beher M. G. Isoprenaline-induced changes in rat parotid and submandibular glands are age- and dosage-dependent. Biochem J. 1984 Jul 1;221(1):15–20. doi: 10.1042/bj2210015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys-Beher M. G., Wells D. J. Metachromatic staining patterns of basic proline-rich proteins from rat and human saliva in sodium dodecyl sulfate-polyacrylamide gels. J Appl Biochem. 1984 Oct-Dec;6(5-6):353–360. [PubMed] [Google Scholar]
- Isemura S., Saitoh E., Sanada K. Fractionation and characterization of basic proline-rich peptides of human parotid saliva and the amino acid sequence of proline-rich peptide P-E. J Biochem. 1982 Jun;91(6):2067–2075. doi: 10.1093/oxfordjournals.jbchem.a133900. [DOI] [PubMed] [Google Scholar]
- Isemura S., Saitoh E., Sanada K. The amino acid sequence of a salivary proline-rich peptide, P-C, and its relation to a salivary proline-rich phosphoprotein, protein C. J Biochem. 1980 Apr;87(4):1071–1077. [PubMed] [Google Scholar]
- Levine M. J., Weill J. C., Ellison S. A. The isolation and analysis of a glycoprotein from parotid saliva. Biochim Biophys Acta. 1969 Aug 12;188(1):165–167. doi: 10.1016/0005-2795(69)90060-9. [DOI] [PubMed] [Google Scholar]
- Mandel I. D., Thompson R. H., Jr, Ellison S. A. Studies on the mucoproteins of human parotid saliva. Arch Oral Biol. 1965 May-Jun;10(3):499–507. doi: 10.1016/0003-9969(65)90116-0. [DOI] [PubMed] [Google Scholar]
- Mehansho H., Carlson D. M. Induction of protein and glycoprotein synthesis in rat submandibular glands by isoproterenol. J Biol Chem. 1983 May 25;258(10):6616–6620. [PubMed] [Google Scholar]
- Muenzer J., Bildstein C., Gleason M., Carlson D. M. Properties of proline-rich proteins from parotid glands of isoproterenol-treated rats. J Biol Chem. 1979 Jul 10;254(13):5629–5634. [PubMed] [Google Scholar]
- Muenzer J., Bildstein C., Gleason M., Carlson D. M. Purification of proline-rich proteins from parotid glands of isoproterenol-treated rats. J Biol Chem. 1979 Jul 10;254(13):5623–5628. [PubMed] [Google Scholar]
- Oppenheim F. G., Hay D. I., Franzblau C. Proline-rich proteins from human parotid saliva. I. Isolation and partial characterization. Biochemistry. 1971 Nov;10(23):4233–4238. doi: 10.1021/bi00799a013. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Porchet N., Richet C., Demeyer D., Roussel P., Degand P. Possible amino acid repetitive sequences in the proline-rich glycoprotein of human parotid saliva. Biochim Biophys Acta. 1983 May 18;744(3):342–348. doi: 10.1016/0167-4838(83)90209-1. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Factors affecting the electrophoretic mobility of the major outer membrane proteins of Escherichia coli in polyacrylamide gels. Biochim Biophys Acta. 1979 Nov 23;581(1):163–178. doi: 10.1016/0005-2795(79)90233-2. [DOI] [PubMed] [Google Scholar]
- Saitoh E., Isemura S., Sanada K. Complete amino acid sequence of a basic proline-rich peptide, P-D, from human parotid saliva. J Biochem. 1983 Feb;93(2):495–502. doi: 10.1093/oxfordjournals.jbchem.a134204. [DOI] [PubMed] [Google Scholar]
- Saitoh E., Isemura S., Sanada K. Complete amino acid sequence of a basic proline-rich peptide, P-F, from human parotid saliva. J Biochem. 1983 Mar;93(3):883–888. doi: 10.1093/jb/93.3.883. [DOI] [PubMed] [Google Scholar]
- Saitoh E., Isemura S., Sanada K. Further fractionation of basic proline-rich peptides from human parotid saliva and complete amino acid sequence of basic proline-rich peptide P-H. J Biochem. 1983 Dec;94(6):1991–1999. doi: 10.1093/oxfordjournals.jbchem.a134553. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Stellner K., Saito H., Hakomori S. I. Determination of aminosugar linkages in glycolipids by methylation. Aminosugar linkages of ceramide pentasaccharides of rabbit erythrocytes and of Forssman antigen. Arch Biochem Biophys. 1973 Apr;155(2):464–472. doi: 10.1016/0003-9861(73)90138-0. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wong R. S., Madapallimattam G., Bennick A. The role of glandular kallikrein in the formation of a salivary proline-rich protein A by cleavage of a single bond in salivary protein C. Biochem J. 1983 Apr 1;211(1):35–44. doi: 10.1042/bj2110035. [DOI] [PMC free article] [PubMed] [Google Scholar]