Abstract
Background
The systemic immune-inflammation index (SII) and trouble sleeping are independent risk factors for nonalcoholic fatty liver disease (NAFLD). Nevertheless, studies investigating the combined effects of the SII and troubled sleeping on NAFLD are lacking. In this study, we investigated the independent relationships and interactions between trouble sleeping and the SII among patients with NAFLD.
Methods
Data from seven survey cycles of the National Health and Nutrition Examination Survey (NHANES) (2005–2018) were analyzed. The SII was obtained by counting platelets, neutrophils, and lymphocytes. NAFLD was diagnosed using the US fatty liver index. Trouble sleeping was diagnosed using a sleep disorder questionnaire. The correlation between trouble sleeping and the SII in NAFLD was investigated using multiple regression analysis, subgroup stratification, interaction tests, and restricted cubic spline, and the presence or absence of additive or multiplicative interactions was determined. Additionally, mediation analyses were performed to explore the role of the SII in mediating the effects of trouble sleeping on NAFLD.
Results
The survey included 10 963 participants. Multivariate logistic regression revealed that SII (OR: 1.21, 95% CI 1.08–1.35) and trouble sleeping (OR: 1.24, 95% CI 1.05–1.47) were positively correlated with NAFLD. For NAFLD, an additive but not multiplicative interaction was noted between the SII and trouble sleeping. The SII partially mediated the association between trouble sleeping and NAFLD, accounting for approximately 3.11% of the total effect (95% CI 0.01–0.05).
Conclusion
The SII and trouble sleeping were independently correlated with NAFLD risk. Furthermore, a combined effect may exist between SII and trouble sleeping, which increases the risk of NAFLD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41043-024-00670-9.
Keywords: Systemic immune-inflammation index, Nonalcoholic fatty liver disease, Trouble sleeping, NHANES
Background
Nonalcoholic fatty liver disease (NAFLD) is a prevalent chronic liver condition that poses a growing public health challenge because of its widespread occurrence [1, 2]. The prevalence of NAFLD among adults (aged ≥ 15 years) is expected to reach 33.5% by 2030 [3]. To date, no effective treatment has been established for NAFLD; hence, appropriate treatment strategies, such as controlling risk factors and lifestyle changes, may be beneficial to prevent the onset of NAFLD.
NAFLD can evolve from basic steatosis to nonalcoholic steatohepatitis, cirrhosis, and finally to hepatocellular carcinoma [4]. This evolution involves complex pathophysiological mechanisms, and some studies have suggested that NAFLD progresses to cirrhosis due to fat deposition in hepatocytes, leading to increased oxidative stress and inflammatory responses [5]. Moreover, oxidative stress drives the development of hepatic lobular inflammation, which is a primary factor in NAFLD progression [6, 7]. Therefore, chronic liver inflammation is a critical factor in NAFLD [6]. The systemic immune-inflammation index (SII) is a recently developed inflammatory marker that reflects both local and overall systemic inflammation [8, 9]. Although previous studies have linked the SII to hepatic steatosis, its association with NAFLD has rarely been explored [10].
Sleep disorders, including sleep apnea, insomnia and restless legs. Evidence suggests that sleep disorders are related to NAFLD, although conclusions remain controversial [11–13]. A decrease in sleep duration and sleep disorders can decrease insulin sensitivity and enhance the release of pro-inflammatory substances, potentially leading to the onset of NAFLD [14, 15]. A cohort study of 143 306 participants found that inadequate sleep duration, but not sleep quality, was associated with NAFLD risk [11]. Nevertheless, several studies have confirmed that sleep disorders are related to NAFLD [13, 16]. Furthermore, a survey of 33 045 patients revealed that those with sleep disorders had a markedly elevated risk of NAFLD, regardless of whether the disorders were combined with sleep apnea [16]. Although numerous studies have explored the link between sleep and NAFLD, most have focused solely on the length of sleep and sleep-related conditions; few have investigated the impact of trouble sleeping on NAFLD.
A recent study found that sleep disruption increases liver inflammation in NAFLD, which in turn affects sleep disruption, with a bidirectional correlation between them [12]. Therefore, a common pathway may exist between SII and troubled sleeping, which contributes to NAFLD. However, prior research has primarily examined the SII and trouble sleeping separately as potential risk factors for NAFLD. To date, no survey has examined whether the SII and trouble sleeping interact synergistically to increase the risk of NAFLD. Therefore, based on data from the National Health and Nutrition Examination Survey (NHANES), we aimed to explore the relationship between the SII and trouble sleeping in NAFLD and assess the impact of the interaction between high SII levels and trouble sleeping on the risk of NAFLD.
Materials and methods
Study population and design
Data used in this study were obtained from the National Center for Health Statistics (NCHS) [17]. This comprehensive cross-sectional survey aimed to explore the relationship between the SII and trouble sleeping in patients with NAFLD and assess the impact of the interaction between high SII levels and trouble sleeping on the risk of NAFLD through a complex multistage sampling design that enabled the results to be generalized to the majority of the population. The NHANES was designed to collect nutritional and health-related data from non-institutionalized civilians in the United States of America [18]. Ethical approval was obtained from the NCHS Ethics Review Board before conducting this study. All study participants provided written informed consent [19].
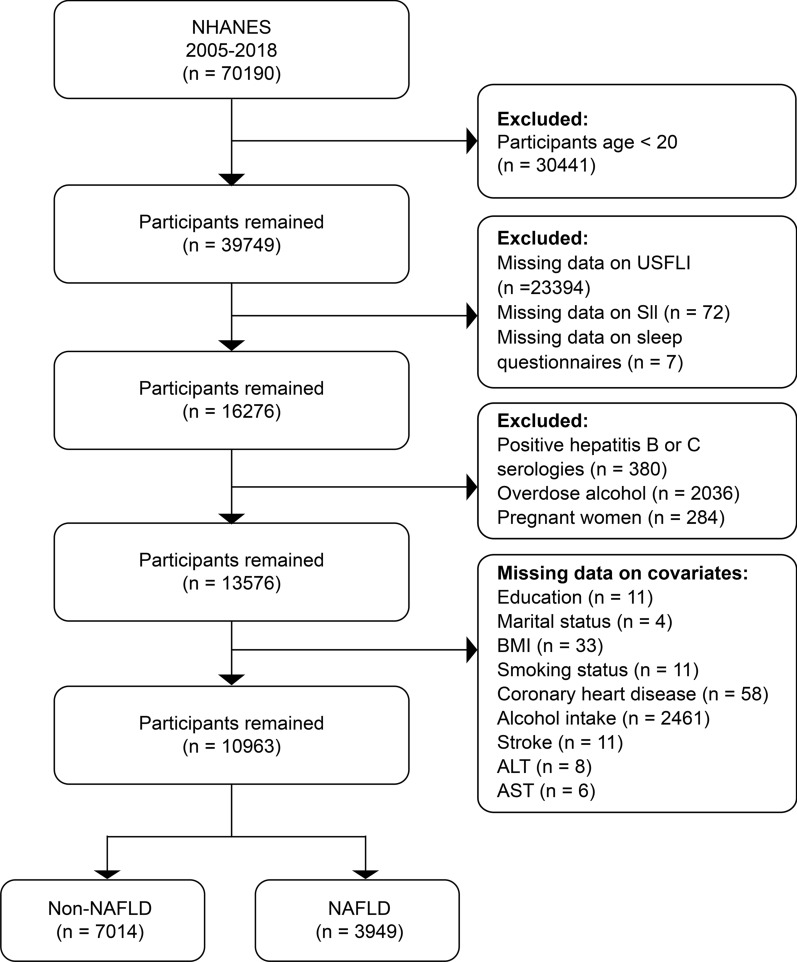
Data from seven 2-year NHANES cycles (2005–2018) were used to collect data for this research (Fig. 1). Notably, data on trouble sleeping were available only for specific cycles. In total, 39 749 participants aged 20 years participated in the survey. Participants were excluded based on the following criteria: (i) missing US fatty liver index (USFLI) data (n = 23 394); (ii) missing SII data (n = 72); (iii) incomplete sleep questionnaire data (n = 7); (iv) viral hepatitis diagnosed by positive hepatitis B surface antigen or hepatitis C antibody (n = 380); (v) alcohol consumption of 30 g/day in men or 20 g/day in women, defined as excessive drinking (n = 2036) [20]; (vi) pregnancy (n = 284); (vii) incomplete covariate data (n = 2589); and (viii) missing data on alanine aminotransferase (ALT) (n = 18) or aspartate aminotransferase (AST) (n = 6). In total, 10,963 participants who met the aforementioned criteria were included in the study.
Fig. 1.
Study design flowchart
SII
The SII is a composite index calculated from a complete blood count test, including lymphocyte, neutrophil, and platelet counts [21]. A Coulter® DxH 800 analyzer was used for automated blood analysis to determine the SII. To calculate the SII, platelet and neutrophil counts were multiplied and the product was divided by the lymphocyte count [8].
Assessment of trouble sleeping
Trouble sleeping was evaluated using a specifically designed questionnaire. The participants were questioned about previous instances in which they had been advised by a healthcare provider or physician regarding trouble sleeping. Participants who answered “yes” were considered to have experienced trouble sleeping, whereas those who answered “no” were not [22].
Definition of NAFLD
The diagnosis of NAFLD was based on the USFLI. Ruhl et al. [23] initially presented this indicator, which was established using NHANESIII data. Individuals were classified as having NAFLD if their USFLI score was ≥ 30 [24]. The USFLI is based on the following formula [23]:
Covariate assessment
To comprehensively assess the potential impact of variables correlated with NAFLD, data on the following sociodemographic characteristics were collected: age, sex (male/female), ethnicity, level of education (greater than high school/less than or equal to high school), marital status (unmarried/married), physical exercise, alcohol use, tobacco use, waist circumference, and body mass index (BMI).
Information on these variables was obtained through in-person interviews conducted in households. Smoking status was classified as never (< 100 cigarettes ever), former (> 100 cigarettes ever but not presently), or current (> 100 cigarettes ever and still smoking). Total energy and alcohol intake were estimated by calculating the mean of two 24-h dietary recall datasets. Physical activity was categorized according to the American Physical Activity Guidelines established by the Department of Health and Human Services. Individuals were classified as physically active if they participated in 150 min of moderate-intensity physical activity per week, whereas those who failed to meet this standard were deemed physically inactive.
Waist circumference was measured in centimeters by drawing a horizontal line above the uppermost lateral margin of the right ilium. In the statistical analyses, waist circumference was classified into four classes (quartiles: Q1: 56.2–88.7 cm; Q2: 88.8–98.8 cm; Q3: 98.9–109.7 cm; and Q4: 109.8–176.0 cm). BMI, calculated by dividing the weight by height squared, was classified into two categories: < 30.0 and ≥ 30.0 [25].
Comorbidities included hypertension, diabetes, stroke, hyperlipidemia, and coronary heart disease. The diagnosis of hypertension included hypertension medication use, self-reported history of hypertension, and an average of three measurements of blood pressure with systolic values of ≥ 130 mmHg or diastolic values of ≥ 80 mmHg [26]. Similarly, diabetes diagnosis relied on self-reported diabetes, antihyperglycemic medication, and fasting blood glucose levels of > 126 mg/dL or glycated hemoglobin levels of ≥ 6.5% [27, 28]. Hyperlipidemia was diagnosed based on high levels of triglyceride (≥ 150 mg/dL), total cholesterol (≥ 200 mg/dL), low-density lipoprotein (≥ 130 mg/dL), High-density lipoprotein (< 40 mg/dL in men or < 50 mg/dL in women), and the use of lipid-lowering medications [29]. Diagnoses of stroke and coronary heart disease were based on self-reported medical histories. The NHANES was performed using a DxC800 system (Beckman Coulter, Brea, CA, USA) using the kinetic rate method for serum ALT and AST levels and the enzyme rate method for gamma-glutamyl transferase activity [30].
Statistical analysis
Weight was considered when conducting the statistical analysis because the NHANES uses a complex sampling research design. The sampling weight used in this study was that of a 7-year mobile examination center. Survey-weighted means were used to express continuous variables, and survey-weighted percentages were used for categorical variables. Chi-square tests were used to compare baseline characteristics between subgroups for categorical variables, and t-tests were used to compare continuous variables. Additionally, the SII data were right-skewed; hence, log2 transformation was performed before statistical analysis. Values converted through calculations were divided into four quartiles, with the lowest quartile used as the reference.
Logistic regression analysis was used to investigate the potential association between the SII and trouble sleeping and an increased risk of NAFLD across models. Additionally, the effect of trouble sleeping on the SII was assessed using a logistic regression model. Model 1 excluded adjusted variables; in Model 2, adjustments were made for age, sex, and race; and Model 3 included covariates such as education level, marital status, physical activity, waist circumference, BMI, smoking habits, total energy intake, alcohol consumption, ALT, AST, gamma-glutamyl transferase (GGT), diabetes, hypertension, hyperlipidemia, history of stroke, and coronary artery disease. Restricted cubic spline regression was used to explore possible nonlinear associations between the SII and NAFLD. Subgroup analyses were conducted to assess the influence of SII and trouble sleeping on NAFLD in various populations, considering factors such as age, sex, race, waist circumference, BMI, diabetes, hypertension, hyperlipidemia, coronary heart disease, and stroke. A stratified analysis of the subgroup variables was conducted using the fully adjusted Model 3. Interaction analysis was used to assess the variability in associations among subgroups.
An additive interaction model was constructed to evaluate whether the coexistence of the SII and trouble sleeping had a greater effect on the risk of NAFLD than the sum of their independent effects [31]. Additive interactions were determined using three metrics: the relative excess risk due to interaction (RERI), attributable proportion (AP), and synergy index (SI). The absence of synergy was indicated by the inclusion of zero in the 95% confidence interval for RERI or AP and the inclusion of one in the 95% confidence interval for SI [32, 33]. The odds ratio (OR) was used to measure the multiplicative interaction between the SII and trouble sleeping and its effect on NAFLD risk. A multiplicative interaction was indicated if the 95% confidence interval (CI) for the product term excluded one. An OR value < 1 obtained by the interaction term indicates the existence of antagonism, and an OR value > 1 obtained by the interaction term indicates synergy [33]. Mediation analysis was performed using the mediation package of R software to determine the extent to which the SII mediates the relationship between trouble sleeping and NAFLD. This is an ideal strategy for elucidating pathways and providing statistical evidence for mechanistic analyses. In this study, the direct effect represents the association between trouble sleeping and NAFLF; the indirect effect, which is the association between trouble sleeping and NAFLF, is mediated by the SII; and the mediation ratio represents the percentage of the mediation effect.
Statistical analysis was conducted using R software version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria). A p-value < 0.05 was set for both groups to determine statistical significance.
Results
Demographic characteristics
Finally, 10,963 participants were selected (weighted n = 65 537 646). Based on the USFLI score, 3949 participants (weighted prevalence of 35.19%) were categorized as having NAFLD, including 2097 males and 1852 females (Table 1). Participants with NAFLD tended to be male and older (aged 52.49 versus [vs.] 46.50 years, respectively), had lower education levels, higher waist circumference, higher BMI, reduced physical activity, were married, and had a history of previous smoking than those without NAFLD. Participants with NAFLD had higher ALT, AST, GGT, and total energy intake than those without NAFLD. In addition, participants with NAFLD were more likely to have metabolic disorders, such as hypertension, hyperlipidemia, and diabetes; a history of coronary heart disease, stroke, and trouble sleeping (34.28% vs. 24.79%); and a higher SII (569.44 vs. 510.84) than participants without NAFLD.
Table 1.
Characteristics of NHANES participants during 2005–2018 based on NAFLD status
| Variables | Overall | NAFLD | p-value | |
|---|---|---|---|---|
| Yes | No | |||
| Age, years | 48.61 (0.28) | 52.49 (0.38) | 46.50 (0.32) | < 0.001 |
| Age, years, n (%) | < 0.001 | |||
| 20–39 | 3430 (33.48) | 871 (23.72) | 2559 (38.78) | |
| 40–59 | 3611 (37.77) | 1366 (39.66) | 2245 (36.74) | |
| ≥ 60 | 3922 (28.74) | 1712 (36.61) | 2210 (24.47) | |
| Sex, n (%) | < 0.001 | |||
| Female | 5820 (52.97) | 1852 (45.03) | 3968 (57.28) | |
| Male | 5143 (47.03) | 2097 (54.97) | 3046 (42.72) | |
| Race/ethnicity, n (%) | < 0.001 | |||
| Non-Hispanic White | 4776 (68.34) | 1834 (71.03) | 2942 (66.89) | |
| Non-Hispanic Black | 2176 (10.24) | 454 (5.79) | 1722 (12.66) | |
| Mexican American | 1771 (8.56) | 944 (12.31) | 827 (6.52) | |
| Other race | 2240 (12.86) | 717 (10.87) | 1523 (13.94) | |
| Marital status, n (%) | < 0.001 | |||
| Married | 8274 (76.07) | 3196 (80.67) | 5087 (73.57) | |
| Unmarried | 2689 (23.93) | 753 (19.33) | 1936 (26.43) | |
| Education, n (%) | < 0.001 | |||
| ≤ High school | 5198 (39.96) | 2130 (45.59) | 3068 (36.91) | |
| > High school | 5765 (60.04) | 1819 (54.41) | 3946 (63.09) | |
| Physical activity, n (%) | < 0.001 | |||
| Yes | 7358 (72.94) | 2414 (67.34) | 4944 (75.97) | |
| No | 3605 (27.06) | 1535 (32.66) | 2070 (24.03) | |
| BMI (kg/m2), n (%) | < 0.001 | |||
| < 30 | 6553 (60.18) | 1155 (26.11) | 5398 (78.68) | |
| ≥ 30 | 4410 (39.81) | 2794 (73.89) | 1616 (21.32) | |
| Waist circumference quartile (cm) | < 0.001 | |||
| Quartile 1 | 2755 (25.96) | 96 (1.69) | 2659 (39.13) | |
| Quartile 2 | 2730 (23.71) | 519 (10.51) | 2211 (30.88) | |
| Quartile 3 | 2741 (24.68) | 1220 (29.14) | 1521 (22.26) | |
| Quartile 4 | 2737 (25.65) | 2114 (58.67) | 623 (7.73) | |
| Smoking status, n (%) | < 0.001 | |||
| Former | 2718 (25.13) | 1214 (31.60) | 1504 (21.62) | |
| Never | 6311 (57.36) | 2100 (52.32) | 4211 (60.10) | |
| Now | 1934 (17.50) | 635 (16.08) | 1299 (18.28) | |
| Hypertension, n (%) | < 0.001 | |||
| Yes | 5845 (49.23) | 2747 (68.99) | 3098 (38.50) | |
| No | 5118 (50.77) | 1202 (31.01) | 3916 (61.50) | |
| Hyperlipidemia, n (%) | < 0.001 | |||
| Yes | 8040 (72.47) | 3448 (87.14) | 4592 (64.50) | |
| No | 2923 (27.53) | 501 (12.86) | 2422 (35.50) | |
| Stroke, n (%) | < 0.001 | |||
| Yes | 469 (3.27) | 209 (4.19) | 260 (2.77) | |
| No | 10 494 (96.73) | 3740 (95.81) | 6754 (97.23) | |
| Coronary heart disease, n (%) | < 0.001 | |||
| Yes | 493 (4.00) | 270 (6.36) | 223 (2.73) | |
| No | 10 470 (96.00) | 3679 (93.64) | 6791 (97.27) | |
| Diabetes, n (%) | < 0.001 | |||
| Yes | 2413 (17.16) | 1542 (33.03) | 871 (8.54) | |
| No | 8550 (82.84) | 2407 (66.97) | 6143 (91.46) | |
| Trouble sleeping, n (%) | < 0.001 | |||
| Yes | 2890 (28.13) | 1250 (34.28) | 1640 (24.79) | |
| No | 8073 (71.87) | 2699 (65.72) | 5374 (75.21) | |
| SII (1,000 cells/µl) | 531.46 (4.25) | 569.44 (6.09) | 510.84 (5.26) | < 0.001 |
| SII Quartile | < 0.001 | |||
| Quartile 1 | 2741 (22.43) | 785 (17.22) | 1956 (25.25) | |
| Quartile 2 | 2741 (25.77) | 981 (24.86) | 1760 (26.28) | |
| Quartile 3 | 2740 (26.10) | 1038 (27.22) | 1702 (25.49) | |
| Quartile 4 | 2741 (25.70) | 1145 (30.70) | 1596 (22.98) | |
| USFLI score | 27.06 (0.38) | 53.83 (0.46) | 12.52 (0.14) | < 0.001 |
| Alanine aminotransferase (U/L) | 24.44 (0.18) | 30.75 (0.38) | 21.02 (0.16) | < 0.001 |
| Aspartate aminotransferase (U/L) | 24.26 (0.16) | 26.47 (0.29) | 23.06 (0.20) | < 0.001 |
| Gamma-glutamyltransferase (U/L) | 26.44 (0.44) | 39.45 (1.08) | 19.37 (0.24) | < 0.001 |
| Total Energy (kcal/day) | 2043.68 (10.88) | 2100.12 (17.63) | 2013.03 (12.10) | < 0.001 |
| Alcohol (g/day) | 3.84 (0.24) | 3.34 (0.32) | 4.11 (0.30) | 0.07 |
Continuous variables are presented as the mean (standard error), and the P-value was determined using the Student’s t-test. Categorical variables are presented as numbers (percentages), and the P-value was determined using the χ2 test
NAFLD: Nonalcoholic fatty liver disease, SII: Systemic immune inflammation index, BMI: Body mass index, NHANES: National Health and Nutrition Examination Survey
Association between SII and NAFLD
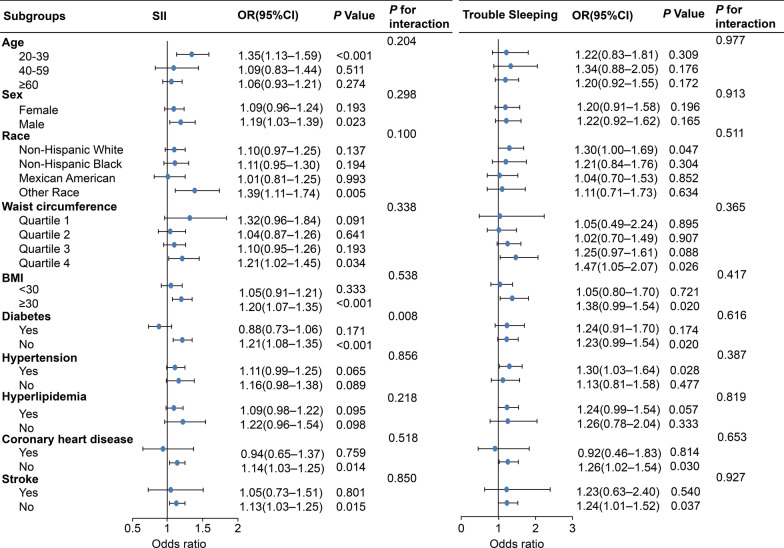
In multivariate logistic regression analysis, the lowest log2-SII level was used as the reference point to assess the correlation between the SII and NAFLD (Additional File 1). When log2SII was expressed as a continuous variable, our findings suggested that a higher SII contributed to increased susceptibility to NAFLD development. This association was significant in Model 1 (OR: 1.39; 95% CI 1.29–1.50) and Model 2 (OR: 1.43; 95% CI 1.31–1.56). The positive correlation between SII and NAFLD remained consistent in Model 3, suggesting that a one-unit increase in the log2-SII score corresponded to a 21% increase in NAFLD risk. The trend p < 0.05 indicated statistical significance for all models. A restricted cubic spline (RCS) analysis was performed to elucidate the nonlinear correlation between the SII and NAFLD (Additional File 2). The weighted RCS analysis indicated no significant nonlinear association between the SII and the risk of developing NAFLD (Poverall < 0.001, Pnon-linear = 0.473). Analyses were conducted in subgroups to study the correlation between the SII and NAFLD across various demographic characteristics and health states while testing for interactions (Fig. 2). A positive association between the SII and NAFLD was observed among individuals aged 20–39 years. In subgroups stratified by sex, the correlation between the SII and NAFLD was significant in the male population. When stratified by BMI or waist circumference, a positive correlation between SII and NAFLD was observed in participants with a BMI ≥ 30 kg/m2 or the largest quartile of waist circumference. For the subgroups stratified by diabetes, stroke, and coronary heart disease, positive associations were observed only among participants without these diseases. However, no correlation was observed in the subgroups stratified by hyperlipidemia or hypertension. Interaction tests showed no significant variations in the relationship between the SII and NAFLD across age, sex, race, waist circumference, BMI, hypertension, hyperlipidemia, stroke, and coronary heart disease, suggesting that these variables did not have a significant impact on this relationship.
Fig. 2.
Forest plot of subgroup analysis between trouble sleeping and systemic immune-inflammation index in NAFLD. Footnotes: Adjusted for age, sex, ethnicity, education, waist circumference, BMI, marital status, smoking status, total energy intake, alcohol intake, physical activity, ALT, AST, GGT, diabetes, hyperlipidemia, hypertension, coronary heart disease, and stroke. Abbreviations: OR: Odds ratio, CI: Confidence interval, SII: Systemic immune-inflammation index, BMI: Body mass index, NAFLD: Nonalcoholic fatty liver disease, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, GGT: Gamma-glutamyl transferase
Association between trouble sleeping and NAFLD
Trouble sleeping was positively associated with NAFLD when no adjustments were made for any variable (Additional file 1). In Model 2, the participants with trouble sleeping had a 1.62-fold increased risk of developing NAFLD. In Model 3, participants with trouble sleeping exhibited a 1.24-fold increased risk of developing NAFLD (OR: 1.24, 95% CI 1.05–1.47). Subgroup analyses were performed in different populations to examine possible links between NAFLD risk and sleeping difficulties (Fig. 2). When stratified by age or sex, the correlation between trouble sleeping and NAFLD did not differ significantly between subgroups. Significant correlations were found between participants with BMIs of ≥ 30 kg/m2 (p < 0.05) but not among those with BMIs of < 30 kg/m2. Similarly, when stratified by waist circumference quartile, a significant positive correlation was observed among participants in the highest waist circumference quartile. Positive associations were observed between participants without stroke or coronary heart disease and those with hypertension. Interaction tests indicated that the link between trouble sleeping and NAFLD remained stable in all strata, demonstrating the robustness of the study results (all p for interaction > 0.05).
Interaction between the SII and trouble sleeping in NAFLD
In the additive interaction model, participants in SII quartiles 1 and 2 were categorized into the normal group, and participants in quartiles 3 and 4 were categorized into the high group. We assessed the existence of additive interactions by calculating the RERI, SI, and AP. The results of the additive interaction model revealed a synergistic effect between high SII and trouble sleeping. In Model 1, the RERI was 2.28 (95% CI 1.46–3.10), AP was 0.56 (95% CI 0.50–0.62), and SI was 3.83 (95% CI 3.31–4.44). These results indicate a synergistic influence of elevated SII combined with trouble sleeping on the risk of developing NAFLD (Table 2). As synergies were still present in Models 2 and 3, the results remained stable (Fig. 3). In Model 3, the AP was 0.27, indicating that 27% of the NAFLD risk in this study sample was caused by interactions between high SII levels and trouble sleeping. No significant multiplicative interaction was identified between SII and trouble sleeping (Model 3, OR: 1.19; 95% CI 0.89–1.59) (Table 2). Logistic regression model analyses showed that trouble sleeping had a significant effect on SII, but the magnitude of the effect varied with model adjustments. Model 1 initially showed that participants with trouble sleeping had a 42.4-fold higher risk of high SII levels than participants without difficulty sleeping (p < 0.001). However, after controlling for additional variables in Model 3, the effect of trouble sleeping on SII levels became non-significant (p = 0.328) (Additional File 3).
Table 2.
Interaction analysis between SII and trouble sleeping in patients with nonalcoholic fatty liver disease
| SII | Trouble sleeping | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Additive interaction | |||||||
|
Normal-level (n = 5483) |
No (n = 8073) |
Ref | Ref | Ref | |||
|
Normal-level (n = 5483) |
Yes (n = 2890) |
1.45 (1.28, 1.65) | < 0.001 | 1.40 (1.20, 1.63) | < 0.001 | 1.02 (0.84, 1.24) | 0.819 |
|
High-level (n = 5480) |
No (n = 8073) |
1.35 (1.23, 1.48) | < 0.001 | 1.32 (1.19, 1.47) | < 0.001 |
1.11 (0.96, 1.27) |
0.149 |
|
High-level (n = 5480) |
Yes (n = 2890) |
2.08 (1.84, 2.34) | < 0.001 |
2.05 (1.77, 2.36 |
< 0.001 | 1.39 (1.13, 1.63) | 0.001 |
| RERI (95% CI) |
2.28 (1.46, 3.10) |
2.07 (1.18, 2.95) | 0.41 (0.05, 0.77) | ||||
| AP (95% CI) | 0.56 (0.50, 0.62) | 0.55 (0.47, 0.62) | 0.27 (0.12, 0.41) | ||||
| SI (95% CI) | 3.83 (3.31, 4.44) | 3.86 (3.22, 4.62) | 4.17 (1.10, 15.77) | ||||
| Multiplicative interaction | |||||||
| SII × Trouble sleeping | 1.06 (0.89, 1.26) | 0.519 | 1.10 (0.90, 1.36) | 0.340 | 1.19 (0.89, 1.59) | 0.237 | |
No adjustments were made to Model 1. Model 2 is adjusted for age, sex, and ethnicity. Model 3 was adjusted for the variables in Model 2, including education, waist circumference, body mass index, marital status, smoking habits, total energy intake, alcohol intake, physical activity, ALT, AST, GGT, diabetes, hyperlipidemia, hypertension, coronary heart disease, and stroke
Abbreviations: SII: Systemic immune-inflammation index, CI: Confidence interval, RERI: Relative excess risk of interaction, AP: Attribution proportion, SI: Synergy index, OR: Odds ratio, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, GGT: Gamma-glutamyl transferase
Fig. 3.
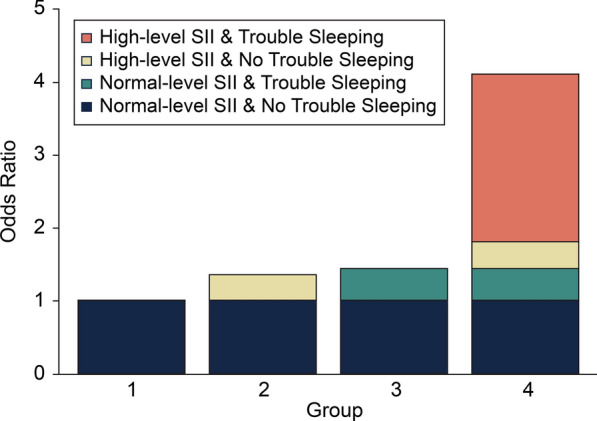
Interaction between SII and trouble sleeping after adjusting for confounders. Footnotes: Adjusted for age, sex, ethnicity, education, waist circumference, BMI, marital status, smoking status, total energy intake, alcohol intake, physical activity, ALT, AST, GGT, diabetes, hyperlipidemia, hypertension, coronary heart disease, and stroke. Abbreviations: SII: Systemic immune inflammation index, BMI: Body mass index, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, GGT: Gamma-glutamyl transferase
Mediation analysis
In the mediation analyses, trouble sleeping, SII, and NAFLD were considered independent, mediating, and dependent variables, respectively. The results showed a significant direct effect of trouble sleeping on NAFLD (β coefficients: 0.095, 95% CI 0.07–0.12) and a significant indirect effect of trouble sleeping on NAFLD through the SII, with an indirect effect size of 0.0311 (p < 0.001) (Additional File 4). This suggests that SII partially mediated the association between trouble sleeping and NAFLD, accounting for approximately 3.11% (95% CI 0.01–0.05) of the total effect; however, the magnitude was much smaller than the direct effect.
Discussion
In this study, we evaluated the association between SII, trouble sleeping, and NAFLD risk based on data from seven NHANES cycles (2005–2018). These findings indicate that SII and trouble sleeping are independently associated with a high risk of developing NAFLD. The results also revealed a synergistic effect of SII and trouble sleeping on NAFLD development. Furthermore, 27% of all patients with NAFLD were affected by the interplay between trouble sleeping and the SII. Additionally, the SII has a mediating role in the positive correlation between trouble sleeping and NAFLD, with a mediation ratio of 3.11%.
The SII is considered a robust indicator of local immune status and overall inflammation in the body [9, 10, 34]. Research has shown that the mechanism underlying NAFLD development is related to insulin resistance and lipotoxicity, which in turn lead to an inflammatory response [35]. This process is believed to be related to elevated neutrophil counts [35]. Indeed, Marques et al. [36] found that increased leukocyte counts, especially neutrophil counts, may increase the risk of developing NAFLD, which is in line with our conclusions. The activation of a range of immune cells and the release of pro-inflammatory factors are also important drivers of NAFLD development [37–39]. Hawkland et al. [40] assessed the histology of blood samples collected from 47 patients with NAFLD. Their results showed significant elevation of several inflammatory factors in patients with NAFLD, which remained high even after adjusting for confounding factors, suggesting that patients with NAFLD have a low-grade systemic inflammatory response [40]. The inflammatory response in NAFLD has also been correlated with several inflammatory markers, including the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and platelet parameters [41–43]. Shavakhi et al. [35] validated the NLR ratio as a predictive marker of NAFLD. An increase in the NLR appears to underlie elevated levels of pro-inflammatory factors, which can be clearly observed from the persistent activation of neutrophils in many patients with NAFLD [41]. Liu et al. [42] investigated the SII, NLR, PLR, and lymphocyte-to-monocyte ratio (LMR) to predict NAFLD risk. Their study confirmed that elevated SII, NLR, and LMR levels were important factors for an increased risk of NAFLD, highlighting the role of systemic immunoinflammatory biomarkers in predicting NAFLD risk [42]. In NAFLD, platelets are highly activated and produce excessive levels of inflammatory cytokines by enhancing thrombosis and promoting an inflammatory response that increases the migration of neutrophils and lymphocytes and induces liver injury [41, 42]. Therefore, the SIIs selected in this study included neutrophils, platelets, and lymphocytes, which could reflect the degree of inflammation and the relationship between the SII and NAFLD.
Recently, improved sleep quality has been suggested to prevent NAFLD [44]. An analysis of 15 studies indicated a significant association between inadequate sleep duration and an increased risk of developing NAFLD, suggesting that adequate sleep may prevent NAFLD [45]. Indeed, Um et al. [46] found that reduced sleep duration or poor sleep quality was linked to a higher risk of NAFLD, emphasizing the importance of sleep quality in reducing the risk of NAFLD [46]. Much of the previous research has focused on evaluating the association between sleep duration, obstructive sleep apnea syndrome, and NAFLD [11, 47, 48]; however, few studies have assessed the impact of trouble sleeping as a major exposure or influencing factor for NAFLD risk. Our findings suggest that patients with trouble sleeping have an increased risk of developing NAFLD. Trouble sleeping may lead to metabolic disorders, such as insulin resistance and fat metabolism disorders, which in turn increase the risk of NAFLD [14]. Trouble sleeping may also interfere with normal neuroendocrine system regulation; this may lead to changes in hormone levels, such as increased cortisol and decreased growth hormone levels, which affects fat metabolism and liver function, thereby increasing the risk of NAFLD [49].
Although the influence of both the SII and trouble sleeping on NAFLD onset is known [11, 50], their synergistic effect remains understudied. The current findings suggest that participants with higher SII scores and trouble sleeping are at higher risk of developing NAFLD. The mechanisms linking trouble sleeping, SII, and NAFLD share several common pathways, including inflammation and the nervous system [50–52]. Inflammatory mediators may link the SII and sleep problems in NAFLD [10]. Trouble sleeping can activate inflammatory cytokines, cause oxidative stress, and promote the creation of an inflammatory microenvironment within the body, which is associated with the development of NAFLD [53]. Trouble sleeping induces Toll-like 4 receptor (TLR-4) activation in monocytes, leading to an increase in inflammatory cell markers such as interleukin-1β, interleukin-6, and interleukin-17 [53]. Inflammatory mediators may be associated with sleep impairment and NAFLD through multiple pathways. First, inflammatory mediators may interfere with normal sleep patterns and rhythms by affecting central nervous system regulation [54]. Second, inflammatory mediators may act directly on the adipose tissue and liver, leading to fat accumulation, hepatic inflammation, and fibrosis, which may further intensify NAFLD [55]. The SII is also a known marker of inflammatory response [8]. Thus, a high SII may indicate increased inflammation in individuals with sleep disorders [56]. Consistent with a previous study [57], the relationship between the SII and trouble sleeping may be bidirectional and synergistic to promote NAFLD development. Second, trouble sleeping may cause overstimulation of the sympathetic nervous system, resulting in elevated cortisol levels and inflammatory marker levels [58]. A population-based longitudinal cohort study showed that autonomic imbalance, particularly overactivation of sympathetic nervous system activity, was strongly associated with the occurrence of NAFLD [52]. Moreover, a study on patients with sleep disorders found significantly higher IL-6 and CRP levels than in normal controls [59]. These inflammatory mediators can directly or indirectly stimulate sympathetic nervous system activity, leading to increased catecholamine neurotransmitter release, which can influence the development of NAFLD [48]. An overactive sympathetic nervous system may be associated with the development and progression of NAFLD [60]. Sympathetic nerve fibers directly innervate and/or are near hepatocytes, hepatic stellate cells, and hepatic sinusoidal endothelial cells and are involved in the regulation of lipid metabolism, processing of very-low-density lipoproteins, and glucose metabolism, all of which are processes that are closely related to the pathogenesis of NAFLD [60]. Hurr et al. [60] attenuated hepatic steatosis by removing hepatic sympathetic nerves using drugs or phenol. Beta-blockers have also been shown to ameliorate hepatic fat deposition in rats with NAFLD [61]. Sympathetic overactivation induced by sleep disorders plays an important role in NAFLD pathogenesis through direct proinflammatory and intensified metabolic disturbances. Further validation of our proposed mechanisms for the interaction between high SII and trouble sleeping will require additional prospective clinical investigations.
A major strength of this study is the use of a comprehensive sample. Additionally, no previous studies have investigated the interaction between SII levels and trouble sleeping and its impact on NAFLD. Our findings suggest that a synergistic effect exists between high SII and trouble sleeping. However, this study has some limitations. Based on the NHANES study design, trouble sleeping diagnosis was obtained using self-reported questionnaires, which introduced recall bias. However, cross-sectional studies are commonly used in large epidemiological surveys, and self-reported sleep questionnaires are used to examine disease associations. Second, cross-sectional study designs cannot assess causal relationships between variables because of their inherent limitations. Therefore, additional research is required to elucidate the causality. Third, we adopted a higher USFLI threshold as an indicator of NAFLD diagnosis. Whereas the use of higher thresholds may result in the exclusion of more samples due to more stringent criteria, this may affect the representativeness of the analyzed samples. Although the use of strict USFLI thresholds to diagnose NAFLD is a limitation of our study, we believe our findings are valuable and provide a useful reference for further research in the field of NAFLD. However, we emphasize the need to address this issue in future studies and conduct more comprehensive analyses to enhance the definition of the diagnostic criteria for NAFLD. Additionally, within the timeframe of this research, the NHANES dataset did not fully cover chronic viral infection screening, particularly viruses that were uncommon or not of concern at the time, which limited patient identification and may have biased the results, affecting the comprehensiveness and accuracy of the conclusions. Although this study revealed the existence of a synergistic interaction between the SII and trouble sleeping, we did not explore possible mediators of this association. Finally, although our model accounted for as many potential confounders as possible, it did not entirely eliminate the impact of other confounding variables, such as Willson’s disease, which may have an impact on study outcomes.
Conclusions
The SII and trouble sleeping were independently associated with an increased risk of NAFLD. A potential combined effect may exist between the SII and trouble sleeping, which increases the risk of NAFLD. Managing inflammatory levels in the body and reducing SII levels, along with ensuring good sleep habits, may help reduce the risk of NAFLD. However, additional large-scale studies are required to confirm these findings.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- SII
Systemic immune-inflammation index
- NHANES
National health and nutrition examination survey
- NCHS
National center for health statistics
- USFLI
US fatty liver index
- BMI
Body mass index
- RERI
Relative excess risk due to interaction
- AP
Attributable proportion
- SI
Synergy index
- OR
Odds ratio
- CI
Confidence interval
- RCS
Restricted cubic spline
- vs.
Versus
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- GGT
Gamma glutamyltransferase
- STAT
Signal transducer and activator of transcription
- TLR-4
Toll-like 4 receptor
- NLR
Neutrophil/lymphocyte ratio
- PLR
Platelet/lymphocyte ratio
- LMR
Lymphocyte-to-monocyte ratio
Author contributions
XY and SZ designed the study and wrote the manuscript; XY and HZ collected, analyzed, and interpreted the data; and TF critically reviewed, edited, and approved the manuscript. All of the authors have read and approved the final version of the manuscript.
Funding
Not applicable.
Data availability
The datasets generated and/or analyzed in the current study are available from the NHANES repository (https://www.cdc.gov/nchs/nhanes/index.htm).
Declarations
Ethical approval and consent to participate
Ethical approval was obtained from the NCHS Ethics Review Board before conducting this study. All the study participants provided written informed consent for their involvement.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335–47. 10.1097/HEP.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–33. 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–64. 10.1016/j.cell.2021.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Fernández-Galilea M, Martínez-Fernández L, González-Muniesa P, Pérez-Chávez A, Martínez JA, et al. Oxidative stress and non-alcoholic fatty liver disease: effects of omega-3 fatty acid supplementation. Nutrients. 2019;11:872. 10.3390/nu11040872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutti S, Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17:81–92. 10.1038/s41575-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meli R, Mattace Raso G, Calignano A. Role of innate immune response in non-alcoholic fatty liver disease: metabolic complications and therapeutic tools. Front Immunol. 2014;5:177. 10.3389/fimmu.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–22. 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 9.Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23:6261–72. 10.3748/wjg.v23.i34.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie R, Xiao M, Li L, Ma N, Liu M, Huang X, et al. Association between SII and hepatic steatosis and liver fibrosis: A population-based study. Front Immunol. 2022;13: 925690. 10.3389/fimmu.2022.925690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Um YJ, Chang Y, Jung HS, Cho IY, Shin JH, Shin H, et al. Sleep duration, sleep quality, and the development of nonalcoholic fatty liver disease: a cohort study. Clin Transl Gastroenterol. 2021;12:00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernsmeier C, Weisskopf DM, Pflueger MO, Mosimann J, Campana B, Terracciano L, et al. Sleep disruption and daytime sleepiness correlating with disease severity and insulin resistance in non-alcoholic fatty liver disease: a comparison with healthy controls. PLoS ONE. 2015;10: e0143293. 10.1371/journal.pone.0143293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi A, Anzai Y, Kuroda M, Kokubun M, Kondo Y, Ogata T, et al. Effects of sleep quality on non-alcoholic fatty liver disease: a cross-sectional survey. BMJ Open. 2020;10: e039947. 10.1136/bmjopen-2020-039947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briançon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr. 2015;7:25. 10.1186/s13098-015-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013;5:93–107. 10.2147/NSS.S31063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei YT, Lee PY, Lin CY, Chen HJ, Lin CC, Wu JS, et al. Non-alcoholic fatty liver disease among patients with sleep disorders: a Nationwide study of Taiwan. BMC Gastroenterol. 2020;20:32. 10.1186/s12876-020-1178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, et al. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital Health Stat 2. 2013;(160):1–23 [PubMed]
- 18.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1. 2013;(56):1–37 [PubMed]
- 19.CDC. National Center for Health Statistics Ethics Review Board (ERB). Available at: https://www.cdc.gov/nchs/nhanes/irba98.html [Accessed on 20 January 2024]
- 20.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 21.Song Y, Guo W, Li Z, Guo D, Li Z, Li Y. Systemic immune-inflammation index is associated with hepatic steatosis: evidence from NHANES 2015–2018. Front Immunol. 2022. 10.3389/fimmu.2022.1058779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai Y, Chen M, Zhai W, Wang C. Interaction between trouble sleeping and depression on hypertension in the NHANES 2005–2018. BMC Public Health. 2022;22:481. 10.1186/s12889-022-12942-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States national health and nutrition examination survey. Aliment Pharmacol Ther. 2015;41:65–76. 10.1111/apt.13012. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Huang Q, Yang L, Zhang R, Gao L, Han X, et al. The association between non-alcoholic fatty liver disease (NAFLD) and advanced fibrosis with serological vitamin B12 markers: results from the NHANES 1999–2004. Nutrients. 2022;14:1224. 10.3390/nu14061224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan J, Hu Y, Pang N, Yang L. Association between dietary niacin Intake and nonalcoholic fatty liver disease: NHANES 2003–2018. Nutrients. 2023;15:4128. 10.3390/nu15194128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Hypertension. 2018;71(6):1269–324. 10.1161/hyp.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 27.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–9. 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 28.Hu XY, Liang YC, Zhang HH, Li HL, Liu DL. Association between the systemic immune-inflammation index and thyroid function in US adults. Mediators Inflamm. 2023;2023:5831858. 10.1155/2023/5831858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kammerlander AA, Mayrhofer T, Ferencik M, Pagidipati NJ, Karady J, Ginsburg GS, et al. Association of metabolic phenotypes with coronary artery disease and cardiovascular events in patients with stable chest pain. Diabetes Care. 2021;44:1038–45. 10.2337/dc20-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) National health and nutrition examination laboratory procedures manual. Hyattsville, MD: United States Department of Health and Human Services, Centers for Disease Control and Prevention; 2009. (Accessed on 21 February 2021)
- 31.Knol MJ, van der Tweel I, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36:1111–8. 10.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- 32.Huang G, Ren G. Interaction between ω-6 fatty acids intake and blood cadmium on the risk of low cognitive performance in older adults from National Health and Nutrition Examination Survey (NHANES) 2011–2014. BMC Geriatr. 2022;22:292. 10.1186/s12877-022-02988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41:514–20. 10.1093/ije/dyr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fest J, Ruiter R, Ikram MA, Voortman T, van Eijck CHJ, Stricker BH. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: a population-based prospective cohort study. Sci Rep. 2018;8:10566. 10.1038/s41598-018-28646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shavakhi M, Nourigheimasi S, Dioso E, Goutnik M, Lucke-Wold B, Khanzadeh S, et al. Prognostic role of neutrophil to lymphocyte ratio in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Can J Gastroenterol Hepatol. 2022;2022:1554079. 10.1155/2022/1554079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marques P, Francisco V, Martínez-Arenas L, Carvalho-Gomes Â, Domingo E, Piqueras L, et al. Overview of cellular and soluble mediators in systemic inflammation associated with non-alcoholic fatty liver disease. Int J Mol Sci. 2023;24:2313. 10.3390/ijms24032313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilg H, Adolph TE, Dudek M, Knolle P. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab. 2021;3:1596–607. 10.1038/s42255-021-00501-9. [DOI] [PubMed] [Google Scholar]
- 38.Huby T, Gautier EL. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol. 2022;22:429–43. 10.1038/s41577-021-00639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heymann F, Tacke F. Immunology in the liver—from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110. 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 40.Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167–74. 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 41.Abdel-Razik A, Mousa N, Shabana W, Refaey M, ElMahdy Y, Elhelaly R, et al. A novel Model using mean platelet volume and neutrophil to lymphocyte ratio as a marker of nonalcoholic steatohepatitis in NAFLD patients: multicentric study. Eur J Gastroenterol Hepatol. 2016;28:e1-9. 10.1097/MEG.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 42.Liu K, Tang S, Liu C, Ma J, Cao X, Yang X, et al. Systemic immune-inflammatory biomarkers (SII, NLR, PLR and LMR) linked to non-alcoholic fatty liver disease risk. Front Immunol. 2024;15:1337241. 10.3389/fimmu.2024.1337241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milovanovic Alempijevic T, Stojkovic Lalosevic M, Dumic I, Jocic N, Pavlovic Markovic A, Dragasevic S, et al. Diagnostic accuracy of platelet count and platelet indices in noninvasive assessment of fibrosis in nonalcoholic fatty liver disease patients. Can J Gastroenterol Hepatol. 2017;2017:6070135. 10.1155/2017/6070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X, Zhuo S, Fang T. Interaction between dietary flavonoid intake and trouble sleeping on non-alcoholic fatty liver disease risk: a cross-sectional study. Eur J Gastroenterol Hepatol. 2024;36:210–9. 10.1097/MEG.0000000000002687. [DOI] [PubMed] [Google Scholar]
- 45.Yang J, Zhang K, Xi Z, Ma Y, Shao C, Wang W, et al. Short sleep duration and the risk of nonalcoholic fatty liver disease/metabolic associated fatty liver disease: a systematic review and meta-analysis. Sleep Breath. 2023;27:1985–96. 10.1007/s11325-022-02767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Um YJ, Chang Y, Jung HS, Cho IY, Shin JH, Shin H, et al. Decrease in sleep duration and poor sleep quality over time is associated with an increased risk of incident non-alcoholic fatty liver disease. J Pers Med. 2022;12:92. 10.3390/jpm12010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Ungprasert P. Short sleep duration and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:1802–7. 10.1111/jgh.13391. [DOI] [PubMed] [Google Scholar]
- 48.Mesarwi OA, Loomba R, Malhotra A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am J Respir Crit Care Med. 2019;199:830–41. 10.1164/rccm.201806-1109TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith PC, Mong JA. Neuroendocrine control of sleep. Curr Top Behav Neurosci. 2019;43:353–78. 10.1007/7854_2019_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao B, Liu Y, Yang Y, He J. Association of systemic immune-inflammation index with non-alcoholic fatty liver disease: a population-based cross-sectional study. Risk Manag Healthc Policy. 2023;16:1581–92. 10.2147/RMHP.S419183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pongratz G, Straub RH. The sympathetic nervous response in inflammation. Arthritis Res Ther. 2014;16:504. 10.1186/s13075-014-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung I, Lee DY, Lee MY, Kwon H, Rhee EJ, Park CY, et al. Autonomic imbalance increases the risk for non-alcoholic fatty liver disease. Front Endocrinol (Lausanne). 2021;12: 752944. 10.3389/fendo.2021.752944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irwin MR, Witarama T, Caudill M, Olmstead R, Breen EC. Sleep loss activates cellular inflammation and signal transducer and activator of transcription (STAT) family proteins in humans. Brain Behav Immun. 2015;47:86–92. 10.1016/j.bbi.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali T, Choe J, Awab A, Wagener TL, Orr WC. Sleep, immunity and inflammation in gastrointestinal disorders. World J Gastroenterol. 2013;19:9231–9. 10.3748/wjg.v19.i48.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease—novel insights into cellular communication circuits. J Hepatol. 2022;77:1136–60. 10.1016/j.jhep.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 56.You Y, Chen Y, Fang W, Li X, Wang R, Liu J, et al. The association between sedentary behavior, exercise, and sleep disturbance: a mediation analysis of inflammatory biomarkers. Front Immunol. 2022;13:1080782. 10.3389/fimmu.2022.1080782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadier K, Dilixiati D, Ainiwaer A, Liu X, Lu J, Liu P, et al. Analysis of the relationship between sleep-related disorder and systemic immune-inflammation index in the US population. BMC Psychiatry. 2023;23:773. 10.1186/s12888-023-05286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carter JR, Grimaldi D, Fonkoue IT, Medalie L, Mokhlesi B, Van Cauter E. Assessment of sympathetic neural activity in chronic insomnia: evidence for elevated cardiovascular risk. Sleep. 2018. 10.1093/sleep/zsy126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferrie JE, Kivimäki M, Akbaraly TN, Singh-Manoux A, Miller MA, Gimeno D, et al. Associations between change in sleep duration and inflammation: findings on C-reactive protein and interleukin 6 in the Whitehall II Study. Am J Epidemiol. 2013;178:956–61. 10.1093/aje/kwt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hurr C, Simonyan H, Morgan DA, Rahmouni K, Young CN. Liver sympathetic denervation reverses obesity-induced hepatic steatosis. J Physiol. 2019;597:4565–80. 10.1113/JP277994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lastuvkova H, Nova Z, Hroch M, Alaei Faradonbeh F, Schreiberova J, Mokry J, et al. Carvedilol impairs bile acid homeostasis in mice: implication for nonalcoholic steatohepatitis. Toxicol Sci. 2023;196:200–17. 10.1093/toxsci/kfad088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed in the current study are available from the NHANES repository (https://www.cdc.gov/nchs/nhanes/index.htm).